Mitochondrial aconitase knockdown attenuates paraquat-induced dopaminergic cell death via decreased cellular metabolism and release of iron and H₂O₂
- PMID: 21517855
- PMCID: PMC3182850
- DOI: 10.1111/j.1471-4159.2011.07290.x
Mitochondrial aconitase knockdown attenuates paraquat-induced dopaminergic cell death via decreased cellular metabolism and release of iron and H₂O₂
Abstract
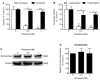
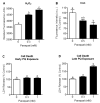
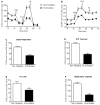
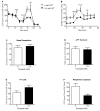
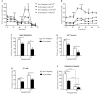
Mitochondrial oxidative stress is a contributing factor in the etiology of numerous neuronal disorders. However, the precise mechanism(s) by which mitochondrial reactive oxygen species modify cellular targets to induce neurotoxicity remains unknown. In this study, we determined the role of mitochondrial aconitase (m-aconitase) in neurotoxicity by decreasing its expression. Incubation of the rat dopaminergic cell line, N27, with paraquat (PQ(2+) ) resulted in aconitase inactivation, increased hydrogen peroxide (H(2) O(2) ) and increased ferrous iron (Fe(2+) ) at times preceding cell death. To confirm the role of m-aconitase in dopaminergic cell death, we knocked down m-aconitase expression via RNA interference. Incubation of m-aconitase knockdown N27 cells with PQ(2+) resulted in decreased H(2) O(2) production, Fe(2+) accumulation, and cell death compared with cells expressing basal levels of m-aconitase. To determine the metabolic role of m-aconitase in mediating neuroprotection, we conducted a complete bioenergetic profile. m-Aconitase knockdown N27 cells showed a global decrease in metabolism (glycolysis and oxygen consumption rates) which blocked PQ(2+) -induced H(+) leak and respiratory capacity deficiency. These findings suggest that dopaminergic cells are protected from death by decreasing release of H(2) O(2) and Fe(2+) in addition to decreased cellular metabolism.
Published 2011. This article is a US Government work and is in the public domain in the USA. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures


References
-
- Arthur CR, Morton SL, Dunham LD, Keeney PM, Bennett JP., Jr. Parkinson’s disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance. Mol Neurodegener. 2009;4:37. - PMC - PubMed
-
- Baron M, Kudin AP, Kunz WS. Mitochondrial dysfunction in neurodegenerative disorders. Biochem Soc Trans. 2007;35:1228–1231. - PubMed
-
- Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998;1366:211–223. - PubMed
-
- Berg D, Youdim MB. Role of iron in neurodegenerative disorders. Top Magn Reson Imaging. 2006;17:5–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

