The influence of interleukin-4 on ligament healing
- PMID: 21518087
- PMCID: PMC3147289
- DOI: 10.1111/j.1524-475X.2011.00682.x
The influence of interleukin-4 on ligament healing
Abstract
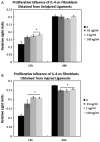
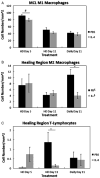
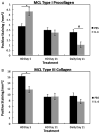
Despite a complex cascade of cellular events to reconstruct the damaged extracellular matrix, ligament healing results in a mechanically inferior scarred ligament. During normal healing, granulation tissue expands into any residual normal ligamentous tissue (creeping substitution), resulting in a larger region of healing, greater mechanical compromise and an inefficient repair process. To control creeping substitution and possibly enhance the repair process, the antiinflammatory cytokine, interleukin-4 (IL-4), was administered to rats before and after rupture of their medial collateral ligaments. In vitro experiments showed a time-dependent effect on fibroblast proliferation after IL-4 treatment. In vivo treatments with IL-4 (100 ng/mL IV) for 5 days resulted in decreased wound size and type III collagen and increased type I procollagen, indicating a more regenerative early healing in response to the IL-4 treatment. However, continued treatment of IL-4 to day 11 antagonized this early benefit and slowed healing. Together, these results suggest that IL-4 not only influences the macrophages and T lymphocytes but also stimulates fibroblasts associated with the proliferative phase of healing in a dose-, cell-, and time-dependent manner. Although treatment significantly influenced healing in the first week after injury, IL-4 alone was unable to maintain this early regenerative response.
© 2011 by the Wound Healing Society.
Figures





References
-
- Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. Journal of cell science. 1995;108 (Pt 3):1251–1261. - PubMed
-
- Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341:738–746. - PubMed
-
- Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. Journal of Biomechanics. 2004;37:865–877. - PubMed
-
- Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27:1392–1398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

