Acceptability of three novel lipid-based nutrient supplements among Malawian infants and their caregivers
- PMID: 21518250
- PMCID: PMC6860564
- DOI: 10.1111/j.1740-8709.2011.00297.x
Acceptability of three novel lipid-based nutrient supplements among Malawian infants and their caregivers
Abstract
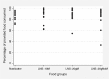
We tested the acceptability of three new lipid-based nutrient supplements (LNSs) in two independent phases among 18 8-12-month-old healthy rural Malawians and their caregivers. In phase 1, acceptability was assessed by offering three new LNSs in random order, and an LNS already determined to be acceptable, Nutributter(®), each added to 30 g of warm maize porridge over three consecutive days. In phase 2, infants from each village were provided one of the new supplements for a 2-week home-use trial. Outcome measures included the amount consumed, time completion of the dose and the maternal rating of likeability on a 5-point scale. The supplements were rated acceptable if consumption was over 50% of the offered dose in phase 1. The mean (95% confidence interval) proportion of the LNS test meals consumed under direct observation was 88% (82-94%) for LNS-10gM, 90% (84-95%) for LNS-20gM, 87% (79-95%) for LNS-20gNoM, and 86% (83-90%) for Nutributter. The median (25th and 75th centile) time (minutes) for completing the offered test meal was 4 (2, 7) for LNS-10gM, 5 (3, 6) for LNS-20gM, 4 (3, 8) for LNS-20gNoM and 4 (2, 6) for Nutributter. During both phases, almost all caregivers rated all study foods very likeable for themselves and their children, with mean scores slightly lower among the caregivers than among the infants. In the home-use phase, the test foods were almost exclusively used by the study participants with minimal sharing with siblings and other household members. Some infants were reported to prefer the new investigational products over traditional complementary food. Considering that the novel LNS was largely acceptable. Efficacy trials are now needed to assess their impact on child growth and development.
© 2011 Blackwell Publishing Ltd.
Conflict of interest statement
Mamane Zeilani is employed by Nutriset S.A.S, a company that produces and sells Plumpy Nut, which is a therapeutic version of LNSs. Other authors declare no conflict of interest. The funders of the trial had no role in the implementation, analysis or reporting.
Figures


References
-
- Adu‐Afarwuah S., Lartey A., Brown K.H., Briend A., Zlotkin S. & Dewey K.G. (2007) Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition 86, 412–420. - PubMed
-
- Barbour R.S. & Kitzinger J. (eds) (1999) Developing Focus Group Research: Politics, Theory and Practice. Sage: London.
-
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. - PubMed
-
- Briend A., Lacsala R., Prudhon C., Mounier B., Grellety Y. & Golden M.H. (1999) Ready‐to‐use therapeutic food for treatment of marasmus. Lancet 353, 1767–1768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

