Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease
- PMID: 21518460
- PMCID: PMC3098801
- DOI: 10.1186/1465-9921-12-55
Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease
Abstract
Background: The long-term efficacy and safety of aclidinium bromide, a novel, long-acting muscarinic antagonist, were investigated in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Methods: In two double-blind, 52-week studies, ACCLAIM/COPD I (n=843) and II (n=804), patients were randomised to inhaled aclidinium 200 μg or placebo once-daily. Patients were required to have a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio of ≤70% and FEV1<80% of the predicted value. The primary endpoint was trough FEV1 at 12 and 28 weeks. Secondary endpoints were health status measured by St George's Respiratory Questionnaire (SGRQ) and time to first moderate or severe COPD exacerbation.
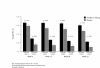
Results: At 12 and 28 weeks, aclidinium improved trough FEV1 versus placebo in ACCLAIM/COPD I (by 61 and 67 mL; both p<0.001) and ACCLAIM/COPD II (by 63 and 59 mL; both p<0.001). More patients had a SGRQ improvement≥4 units at 52 weeks with aclidinium versus placebo in ACCLAIM/COPD I (48.1% versus 39.5%; p=0.025) and ACCLAIM/COPD II (39.0% versus 32.8%; p=0.074). The time to first exacerbation was significantly delayed by aclidinium in ACCLAIM/COPD II (hazard ratio [HR] 0.7; 95% confidence interval [CI] 0.55 to 0.92; p=0.01), but not ACCLAIM/COPD I (HR 1.0; 95% CI 0.72 to 1.33; p=0.9). Adverse events were minor in both studies.
Conclusion: Aclidinium is effective and well tolerated in patients with moderate to severe COPD.
Trial registration: ClinicalTrials.gov: NCT00363896 (ACCLAIM/COPD I) and NCT00358436 (ACCLAIM/COPD II).
Figures


References
-
- Barnes PJ. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med. 2004;117(Suppl 12A):24S–32S. - PubMed
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. - DOI - PubMed
-
- Gavalda A, Miralpeix M, Ramos I, Otal R, Carreno C, Vinals M, Domenech T, Carcasona C, Reyes B, Vilella D, Gras J, Cortijo J, Morcillo E, Llenas J, Ryder H, Beleta J. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther. 2009;331:740–751. doi: 10.1124/jpet.109.151639. - DOI - PubMed
-
- Sentellas S, Ramos I, Alberti J, Salva M, Anton F, Miralpeix M, Beleta J, Gavalda A. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: In vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39:283–290. doi: 10.1016/j.ejps.2010.01.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

