Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis
- PMID: 21518837
- PMCID: PMC3122391
- DOI: 10.1128/AAC.01797-10
Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis
Abstract
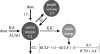
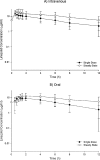
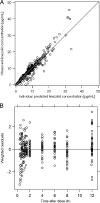
Linezolid is a treatment option for methicillin-resistant Staphylococcus aureus (MRSA) infections in cystic fibrosis (CF) patients. Little is known, however, about its pharmacokinetics in this population. Eight adults with CF were randomized to receive intravenous (i.v.) and oral linezolid at 600 mg twice daily for 9 doses in a crossover design with a 9-day washout. Plasma samples were collected after the first and ninth doses of each phase. Population pharmacokinetic analyses were performed by nonlinear mixed-effects modeling using a previously described 2-compartment model with time-dependent clearance inhibition. Monte Carlo simulation was performed to assess the activities of the linezolid dosing regimens against 42 contemporary MRSA isolates recovered from CF patients. The following pharmacokinetic parameter estimates were observed for the population: absorption rate constant, 1.91 h(-1); clearance, 9.54 liters/h; volume of central compartment, 26.8 liters; volume of peripheral compartment, 17.3 liters; and intercompartmental clearance, 104 liters/h. Linezolid demonstrated nonlinear clearance after 9 doses, which was reduced by a mean of 38.9% (range, 28.8 to 59.9%). Mean bioavailability was 85% (range, 47 to 131%). At steady state, 600 mg given twice daily produced 93.0% and 87.2% probabilities of obtaining the target pharmacodynamic exposure against the MRSA isolates for the i.v. and oral formulations, respectively. Thrice-daily dosing increased the probabilities to 97.0% and 95.6%, respectively. Linezolid pharmacokinetics in these adults with CF were well described by a 2-compartment model with time-dependent clearance inhibition. Standard i.v. and oral dosing regimens should be sufficient to reliably attain pharmacodynamic targets against most MRSA isolates; however, more frequent dosing may be required for isolates with MICs of ≥ 2 μg/ml.
Figures

References
-
- Brickner S. J., Barbachyn M. R., Hutchinson D. K., Manninen P. R. 2008. Linezolid (Zyvox), the first member of a completely new class of antibacterial agents for treatment of gram-positive infections. J. Med. Chem. 51:1981–1990 - PubMed
-
- Buerger C., Joukhadar C., Muller M., Kloft C. 2003. Development of a liquid chromatography method for the determination of linezolid and its application to in vitro and human microdialysis samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796:155–164 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

