Identification of a myeloid-derived suppressor cell cystatin-like protein that inhibits metastasis
- PMID: 21518852
- PMCID: PMC3136340
- DOI: 10.1096/fj.10-180604
Identification of a myeloid-derived suppressor cell cystatin-like protein that inhibits metastasis
Abstract
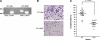
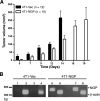
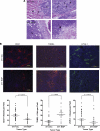
Myeloid-derived suppressor cells (MDSCs) are significantly increased in cancer patients and tumor bearing-animals. MDSCs infiltrate into tumors and promote tumor invasion and metastasis. To identify the mediator responsible for the prometastatic property of MDSCs, we used proteomics. We found neutrophilic granule protein (NGP) was decreased >2-fold in MDSCs from metastatic 4T1 tumor-bearing mice compared to nonmetastatic 67NR controls. NGP mRNA levels were decreased in bone marrow and in tumor-infiltrating MDSCs by 45 and 66%, respectively, in 4T1 tumor-bearing mice compared to 67NR controls. Interestingly, 4T1-conditioned medium reduced myeloid cell NGP expression by ∼ 40%, suggesting that a secreted factor mediates gene reduction. Sequence analysis shows a putative cystatin domain in NGP, and biochemical analysis confirms NGP a novel cathepsin inhibitor. It inhibited cathepsin B activity by nearly 40% in vitro. NGP expression in 4T1 tumor cells suppressed cell invasion, delayed primary tumor growth, and greatly reduced lung metastasis in vivo. A 2.8-fold reduction of cathepsin activity was found in tumors expressing NGP compared to controls. NGP significantly reduced tumor angiogenesis to 12.6 from 19.6 and lymphangiogenesis to 4.6 from 9.1 vessels/field. Necrosis was detectable only in NGP-expressing tumors, and the number of apoptotic cells increased to 22.4 from 8.3 in controls. Taken together, this study identifies a negative regulator of tumor metastasis in MDSCs, NGP, which is down-regulated in metastatic conditions. The finding suggests that malignant tumors promote invasion/metastasis not only through up-regulation of proteases but also down-regulation of protease inhibitors.
Figures

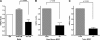
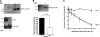


References
-
- Almand B., Clark J. I., Nikitina E., van Beynen J., English N. R., Knight S. C., Carbone D. P., Gabrilovich D. I. (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer J. Immunol. 166, 678–689 - PubMed
-
- Yang L., DeBusk L. M., Fukuda K., Fingleton B., Green-Jarvis B., Shyr Y., Matrisian L. M., Carbone D. P., Lin P. C. (2004) Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 - PubMed
-
- Diaz-Montero C. M., Salem M. L., Nishimura M. I., Garrett-Mayer E., Cole D. J., Montero A. J. (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 - PMC - PubMed
-
- Shojaei F., Wu X., Malik A. K., Zhong C., Baldwin M. E., Schanz S., Fuh G., Gerber H.-P., Ferrara N. (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 25, 911–920 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

