IFN-lambda determines the intestinal epithelial antiviral host defense
- PMID: 21518880
- PMCID: PMC3093475
- DOI: 10.1073/pnas.1100552108
IFN-lambda determines the intestinal epithelial antiviral host defense
Abstract
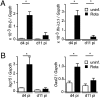
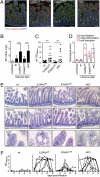
Type I and type III IFNs bind to different cell-surface receptors but induce identical signal transduction pathways, leading to the expression of antiviral host effector molecules. Despite the fact that type III IFN (IFN-λ) has been shown to predominantly act on mucosal organs, in vivo infection studies have failed to attribute a specific, nonredundant function. Instead, a predominant role of type I IFN was observed, which was explained by the ubiquitous expression of the type I IFN receptor. Here we comparatively analyzed the role of functional IFN-λ and type I IFN receptor signaling in the innate immune response to intestinal rotavirus infection in vivo, and determined viral replication and antiviral gene expression on the cellular level. We observed that both suckling and adult mice lacking functional receptors for IFN-λ were impaired in the control of oral rotavirus infection, whereas animals lacking functional receptors for type I IFN were similar to wild-type mice. Using Mx1 protein accumulation as marker for IFN responsiveness of individual cells, we demonstrate that intestinal epithelial cells, which are the prime target cells of rotavirus, strongly responded to IFN-λ but only marginally to type I IFN in vivo. Systemic treatment of suckling mice with IFN-λ repressed rotavirus replication in the gut, whereas treatment with type I IFN was not effective. These results are unique in identifying a critical role of IFN-λ in the epithelial antiviral host defense.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


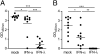
References
-
- Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407–422. - PubMed
-
- Kotenko SV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. - PubMed
-
- Sheppard P, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. - PubMed
-
- Onoguchi K, et al. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282:7576–7581. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

