Structural mechanism of the ATP-induced dissociation of rigor myosin from actin
- PMID: 21518908
- PMCID: PMC3093495
- DOI: 10.1073/pnas.1018420108
Structural mechanism of the ATP-induced dissociation of rigor myosin from actin
Abstract
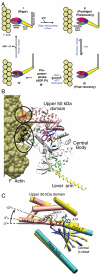
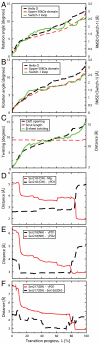
Myosin is a true nanomachine, which produces mechanical force from ATP hydrolysis by cyclically interacting with actin filaments in a four-step cycle. The principle underlying each step is that structural changes in separate regions of the protein must be mechanically coupled. The step in which myosin dissociates from tightly bound actin (the rigor state) is triggered by the 30 Å distant binding of ATP. Large conformational differences between the crystal structures make it difficult to perceive the coupling mechanism. Energetically accessible transition pathways computed at atomic detail reveal a simple coupling mechanism for the reciprocal binding of ATP and actin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Structural characterization of the binding of Myosin*ADP*Pi to actin in permeabilized rabbit psoas muscle.Biophys J. 2006 Nov 1;91(9):3370-82. doi: 10.1529/biophysj.106.086918. Epub 2006 Aug 11. Biophys J. 2006. PMID: 16905611 Free PMC article.
-
Lever arm model of force generation by actin-myosin-ATP.Biochemistry. 1999 Aug 3;38(31):9791-7. doi: 10.1021/bi9907633. Biochemistry. 1999. PMID: 10433684 Review. No abstract available.
-
Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide.Nature. 2003 Sep 25;425(6956):423-7. doi: 10.1038/nature02005. Nature. 2003. PMID: 14508495
-
A new structural state of myosin.Trends Biochem Sci. 2004 Mar;29(3):103-6. doi: 10.1016/j.tibs.2004.01.001. Trends Biochem Sci. 2004. PMID: 15055201 Review.
-
Chemical decoupling of ATPase activation and force production from the contractile cycle in myosin by steric hindrance of lever-arm movement.Biophys J. 2003 Feb;84(2 Pt 1):1047-56. doi: 10.1016/S0006-3495(03)74921-2. Biophys J. 2003. PMID: 12547786 Free PMC article.
Cited by
-
Drosophila myosin mutants model the disparate severity of type 1 and type 2B distal arthrogryposis and indicate an enhanced actin affinity mechanism.Skelet Muscle. 2020 Aug 15;10(1):24. doi: 10.1186/s13395-020-00241-6. Skelet Muscle. 2020. PMID: 32799913 Free PMC article.
-
ATP Analogues for Structural Investigations: Case Studies of a DnaB Helicase and an ABC Transporter.Molecules. 2020 Nov 12;25(22):5268. doi: 10.3390/molecules25225268. Molecules. 2020. PMID: 33198135 Free PMC article. Review.
-
Switch II mutants reveal coupling between the nucleotide- and actin-binding regions in myosin V.Biophys J. 2012 Jun 6;102(11):2545-55. doi: 10.1016/j.bpj.2012.04.025. Epub 2012 Jun 5. Biophys J. 2012. PMID: 22713570 Free PMC article.
-
Catalytic strategy used by the myosin motor to hydrolyze ATP.Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):E2947-56. doi: 10.1073/pnas.1401862111. Epub 2014 Jul 8. Proc Natl Acad Sci U S A. 2014. PMID: 25006262 Free PMC article.
-
How myosin motors power cellular functions: an exciting journey from structure to function: based on a lecture delivered at the 34th FEBS Congress in Prague, Czech Republic, July 2009.FEBS J. 2012 Feb;279(4):551-62. doi: 10.1111/j.1742-4658.2011.08449.x. Epub 2012 Jan 9. FEBS J. 2012. PMID: 22171985 Free PMC article.
References
-
- Kolomeisky AB, Fisher ME. Molecular motors: A theorist’s perspective. Annu Rev Phys Chem. 2007;58:675–695. - PubMed
-
- van den Heuvel MG, Dekker C. Motor proteins at work for nanotechnology. Science. 2007;317:333–336. - PubMed
-
- Itakura S, et al. Force-generating domain of myosin motor. Biochem Biophys Res Commun. 1993;196:1504–1510. - PubMed
-
- Finlayson B, Lymn RW, Taylor EW. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969;8:811–819. - PubMed
-
- Conibear PB, Bagshaw CR, Fajer PG, Kovacs M, Malnasi-Csizmadia A. Myosin cleft movement and its coupling to actomyosin dissociation. Nat Struct Biol. 2003;10:831–835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

