Structural mechanism of the ATP-induced dissociation of rigor myosin from actin
- PMID: 21518908
- PMCID: PMC3093495
- DOI: 10.1073/pnas.1018420108
Structural mechanism of the ATP-induced dissociation of rigor myosin from actin
Abstract
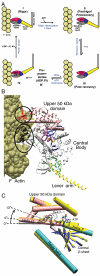
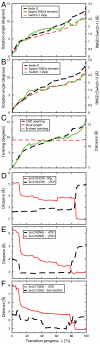
Myosin is a true nanomachine, which produces mechanical force from ATP hydrolysis by cyclically interacting with actin filaments in a four-step cycle. The principle underlying each step is that structural changes in separate regions of the protein must be mechanically coupled. The step in which myosin dissociates from tightly bound actin (the rigor state) is triggered by the 30 Å distant binding of ATP. Large conformational differences between the crystal structures make it difficult to perceive the coupling mechanism. Energetically accessible transition pathways computed at atomic detail reveal a simple coupling mechanism for the reciprocal binding of ATP and actin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kolomeisky AB, Fisher ME. Molecular motors: A theorist’s perspective. Annu Rev Phys Chem. 2007;58:675–695. - PubMed
-
- van den Heuvel MG, Dekker C. Motor proteins at work for nanotechnology. Science. 2007;317:333–336. - PubMed
-
- Itakura S, et al. Force-generating domain of myosin motor. Biochem Biophys Res Commun. 1993;196:1504–1510. - PubMed
-
- Finlayson B, Lymn RW, Taylor EW. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969;8:811–819. - PubMed
-
- Conibear PB, Bagshaw CR, Fajer PG, Kovacs M, Malnasi-Csizmadia A. Myosin cleft movement and its coupling to actomyosin dissociation. Nat Struct Biol. 2003;10:831–835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

