Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma
- PMID: 21519015
- PMCID: PMC3107750
- DOI: 10.1200/JCO.2010.33.9432
Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma
Abstract
PURPOSE Hepatocellular carcinoma (HCC) is a common and deadly malignancy with few systemic therapy options. The RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-related kinase (ERK) pathway is activated in approximately 50% to 60% of HCCs and represents a potential target for therapy. Selumetinib is an orally available inhibitor of MEK tyrosine kinase activity. PATIENTS AND METHODS Patients with locally advanced or metastatic HCC who had not been treated with prior systemic therapy were enrolled on to the study. Patients were treated with selumetinib at its recommended phase II dose of 100 mg twice per day continuously. Cycle length was 21 days. Imaging was performed every two cycles. Biopsies were obtained at baseline and at steady-state in a subset of patients, and pharmacokinetic (PK) analysis was performed on all patients. Results Nineteen patients were enrolled, 17 of whom were evaluable for response. Most (82%) had Child-Pugh A cirrhosis. Toxicity was in line with other studies of selumetinib in noncirrhotic patients. PK parameters were also comparable to those in noncirrhotic patients. No radiographic response was observed in this group, and the study was stopped at the interim analysis. Of 11 patients with elevated α-fetoprotein, three (27%) had decreases of 50% or more. Median time to progression was 8 weeks. Inhibition of ERK phosphorylation was demonstrated by Western blotting. CONCLUSION In this study of selumetinib for patients with HCC, no radiographic responses were seen and time to progression was short, which suggests minimal single-agent activity despite evidence of suppression of target activation.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

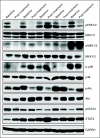
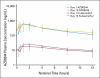
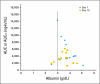
Comment in
-
An uphill battle downstream of RAF.J Clin Oncol. 2011 Jun 10;29(17):2298-300. doi: 10.1200/JCO.2011.34.9316. Epub 2011 Apr 25. J Clin Oncol. 2011. PMID: 21519020 No abstract available.
References
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Blum R, Cox AD, Kloog Y. Inhibitors of chronically active RAS: Potential for treatment of human malignancies. Recent Pat Anticancer Drug Discov. 2008;3:31–47. - PubMed
-
- Avruch J. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem. 1998;182:31–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

