The potential dual effects of anesthetic isoflurane on hypoxia-induced caspase-3 activation and increases in β-site amyloid precursor protein-cleaving enzyme levels
- PMID: 21519046
- PMCID: PMC3123429
- DOI: 10.1213/ANE.0b013e3182185fee
The potential dual effects of anesthetic isoflurane on hypoxia-induced caspase-3 activation and increases in β-site amyloid precursor protein-cleaving enzyme levels
Abstract
Background: β-Amyloid protein (Aβ) accumulation, caspase activation, apoptosis, and hypoxia-induced neurotoxicity have been suggested to be involved in Alzheimer disease neuropathogenesis. Aβ is produced from amyloid precursor protein through proteolytic processing by the aspartyl protease β-site amyloid precursor protein-cleaving enzyme (BACE) and γ-secretase. Inhaled anesthetics have long been considered to protect against neurotoxicity. However, recent studies have suggested that the inhaled anesthetic isoflurane may promote neurotoxicity by inducing caspase activation and apoptosis, and by increasing levels of BACE and Aβ. We therefore sought to determine whether isoflurane can induce concentration-dependent dual effects on hypoxia-induced caspase-3 activation and increases in BACE levels: protection versus promotion.
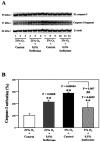
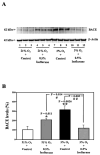
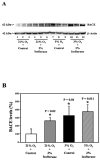
Methods: H4 human neuroglioma cells were treated with hypoxia (3% O(2)) alone, different concentrations of isoflurane (0.5% and 2%), and the combination of hypoxia and 0.5% or 2% isoflurane. The levels of caspase-3 cleavage (activation), BACE, and Bcl-2 were determined by Western blot analysis.
Results: We show for the first time that treatment with 0.5% isoflurane for 8 hours attenuated, whereas treatment with 2% isoflurane for 8 hours enhanced, hypoxia-induced caspase-3 activation and increases in BACE levels. The 2% isoflurane treatment also enhanced a hypoxia-induced decrease in Bcl-2 levels.
Conclusions: These results suggest a potential concept that isoflurane has dual effects (protection versus promotion) on hypoxia-induced toxicity, which may act through Bcl-2 family proteins. These findings could lead to more systematic studies to determine the potential dual effects of anesthetics on Alzheimer disease-associated neurotoxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Tanzi RE, Bertram L. Alzheimer’s disease: The latest suspect. Nature. 2008;454:706–8. - PubMed
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–55. - PubMed
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. - PubMed
-
- Xie Z, Tanzi RE. Alzheimer’s disease and post-operative cognitive dysfunction. Exp Gerontol. 2006;41:346–59. - PubMed
-
- Mattson MP. Contributions of mitochondrial alterations, resulting from bad genes and a hostile environment, to the pathogenesis of Alzheimer’s disease. Int Rev Neurobiol. 2002;53:387–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

