Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations
- PMID: 21519145
- PMCID: PMC3083781
- DOI: 10.1172/JCI46307
Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations
Abstract
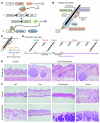
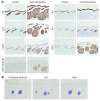
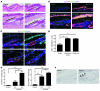
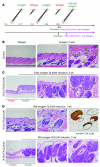
Uncontrolled Hedgehog (Hh) signaling leads to the development of basal cell carcinoma (BCC), the most common human cancer, but the cell of origin for BCC is unclear. While Hh pathway dysregulation is common to essentially all BCCs, there exist multiple histological subtypes, including superficial and nodular variants, raising the possibility that morphologically distinct BCCs may arise from different cellular compartments in skin. Here we have shown that induction of a major mediator of Hh signaling, GLI2 activator (GLI2ΔN), selectively in stem cells of resting hair follicles in mice, induced nodular BCC development from a small subset of cells in the lower bulge and secondary hair germ compartments. Tumorigenesis was markedly accelerated when GLI2ΔN was induced in growing hair follicles. In contrast, induction of GLI2ΔN in epidermis led to the formation of superficial BCCs. Expression of GLI2ΔN at reduced levels in mice yielded lesions resembling basaloid follicular hamartomas, which have previously been linked to low-level Hh signaling in both mice and humans. Our data show that the cell of origin, tissue context (quiescent versus growing hair follicles), and level of oncogenic signaling can determine the phenotype of Hh/Gli-driven skin tumors, with high-level signaling required for development of superficial BCC-like tumors from interfollicular epidermis and nodular BCC-like tumors from hair follicle stem cells.
Figures



Comment in
-
Mommy - where do tumors come from?J Clin Invest. 2011 May;121(5):1681-3. doi: 10.1172/JCI57700. Epub 2011 Apr 25. J Clin Invest. 2011. PMID: 21519146 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

