Individually tailored treatment of medication nonadherence
- PMID: 21519282
- PMCID: PMC3674887
- DOI: 10.1097/MPG.0b013e3182203a91
Individually tailored treatment of medication nonadherence
Abstract
Objective: Nonadherence is a significant health care issue in pediatric inflammatory bowel disease (IBD) that requires intervention to improve outcomes. This pilot randomized controlled trial was designed to evaluate the feasibility, acceptability, and preliminary efficacy of an individually tailored behavioral treatment for nonadherence in adolescents with IBD.
Patients and methods: Fourteen adolescents ages 14.89 ± 2.01 years were randomly assigned to immediate care or wait list control conditions and received a manualized individually tailored behavioral intervention for nonadherence. Medication adherence, measured by pill count, served as the primary endpoint. Parents provided demographic data and ratings of intervention acceptability and patients provided disease-severity data.
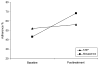
Results: Feasibility of the treatment was demonstrated by 100% treatment session attendance for all of the patients enrolled in the trial. Both parent and patient acceptability ratings were favorable. Comparison of baseline with posttreatment percent adherence across both conditions demonstrated that treatment resulted in a 4% gain in 6-mercaptopurine/azathioprine adherence (52% at baseline; 56% at posttreatment; δ = 0.07) and a 25% gain in mesalamine adherence (43% at baseline; 68% at posttreatment; δ = 0.57).
Conclusions: Individually tailored treatment of nonadherence in adolescents with IBD is feasible and may result in substantial improvement in medication adherence. Differential effect of the intervention on medications requires further investigation, but it may reflect differences in regimen complexity, concerns about medication adverse effects, and/or patient/parent preference to target more complex regimens. Large-scale testing of this intervention is needed to demonstrate effect on clinical outcomes.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Rapoff MA. Adherence to Pediatric Medical Regimens. 2. New York: Springer; 2010.
-
- DiMatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns. 2004;55:339–44. - PubMed
-
- Berg JS, Dischler J, Wagner DJ, et al. Medication compliance: a healthcare problem. Ann Pharmacother. 1993;279(Suppl):S1–24. - PubMed
-
- Logan D, Zelikovsky N, Labay L, et al. The Illness Management Survey: identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources