IL-6 promotes acute and chronic inflammatory disease in the absence of SOCS3
- PMID: 21519345
- PMCID: PMC3146962
- DOI: 10.1038/icb.2011.29
IL-6 promotes acute and chronic inflammatory disease in the absence of SOCS3
Abstract
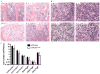
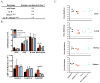
The lack of expression of the suppressor of cytokine signalling-3 (SOCS3) or inactivation of the negative regulatory capacity of SOCS3 has been well documented in rheumatoid arthritis, viral hepatitis and cancer. The specific qualitative and quantitative consequences of SOCS3 deficiency on interleukin-6 (IL-6)-mediated pro- and anti-inflammatory responses remain controversial in vitro and unknown in vivo. Mice with a conditional deletion of SOCS3 in hematopoietic cells develop lethal inflammatory disease during adult life and develop gross histopathological changes during experimental arthritis, typified by elevated IL-6 levels. To clarify the nature of the IL-6 responses in vivo, we generated mice deficient in SOCS3 (SOCS3(-/Δvav)) or both SOCS3 and IL-6 (IL-6(-/-)/SOCS3(-/Δvav)), and examined responses in models of acute and chronic inflammation. Acute responses to IL-1β were lethal to SOCS3(-/Δvav) mice but not IL-6(-/-)/SOCS3(-/Δvav) mice, indicating that IL-6 was required for the lethal inflammation induced by IL-1β. Administration of IL-1β to SOCS3(-/Δvav) mice induced systemic apoptosis of lymphocytes in the thymus, spleen and lymph nodes that was dependent on the presence of IL-6. IL-6 deficiency prolonged survival of SOCS3(-/Δvav) mice and ameliorated spontaneous inflammatory disease developing during adult life. Infection of SOCS3(-/Δvav) mice with LCMV induced a lethal inflammatory response that was dependent on IL-6, despite SOCS3(-/Δvav) mice controlling viral replication. We conclude that SOCS3 is required for survival during inflammatory responses and is a critical regulator of IL-6 in vivo.
Conflict of interest statement
The authors declare no financial conflicts of interest.
Figures
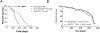

References
-
- Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood. 1997;90:2583–90. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. - PubMed
-
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. - PubMed
-
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

