The nitrosocarbonyl hetero-Diels-Alder reaction as a useful tool for organic syntheses
- PMID: 21520360
- PMCID: PMC4079088
- DOI: 10.1002/anie.201005764
The nitrosocarbonyl hetero-Diels-Alder reaction as a useful tool for organic syntheses
Abstract
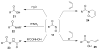
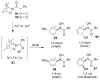
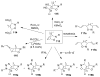
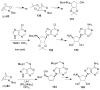
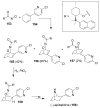
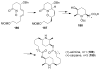
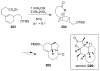
Organic transformations that result in the formation of multiple covalent bonds within the same reaction are some of the most powerful tools in synthetic organic chemistry. Nitrosocarbonyl hetero-Diels-Alder (HDA) reactions allow for the simultaneous stereospecific introduction of carbon-nitrogen and carbon-oxygen bonds in one synthetic step, and provide direct access to 3,6-dihydro-1,2-oxazines. This Review describes the development of the nitrosocarbonyl HDA reaction and the utility of the resulting oxazine ring in the synthesis of a variety of important, biologically active molecules.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


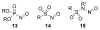



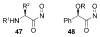







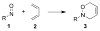





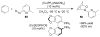





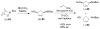






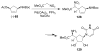






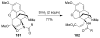
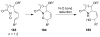


Similar articles
-
Hetero-Diels-Alder reaction of phosphorylated nitroso alkenes with enol ethers on water: a clean approach toward 1,2-oxazine derivatives.J Org Chem. 2014 Aug 15;79(16):7607-15. doi: 10.1021/jo501339c. Epub 2014 Jul 31. J Org Chem. 2014. PMID: 25062501
-
Solid-supported nitroso hetero-Diels-Alder reactions. 3. Acid-mediated transformation of cycloadducts by scission of the oxazine C-O bonds.J Comb Chem. 2008 Jan-Feb;10(1):112-7. doi: 10.1021/cc700142d. Epub 2007 Dec 8. J Comb Chem. 2008. PMID: 18067270
-
Hetero-Diels-Alder reaction of phosphinyl and phosphonyl nitroso alkenes with conjugated dienes: an aza-Cope rearrangement.J Org Chem. 2011 Aug 19;76(16):6715-25. doi: 10.1021/jo201116u. Epub 2011 Jul 15. J Org Chem. 2011. PMID: 21732627
-
The asymmetric hetero-Diels-Alder reaction in the syntheses of biologically relevant compounds.Angew Chem Int Ed Engl. 2014 Oct 13;53(42):11146-57. doi: 10.1002/anie.201404094. Epub 2014 Sep 12. Angew Chem Int Ed Engl. 2014. PMID: 25220929 Review.
-
Stereo- and regioselectivity of the hetero-Diels-Alder reaction of nitroso derivatives with conjugated dienes.Beilstein J Org Chem. 2016 Sep 1;12:1949-1980. doi: 10.3762/bjoc.12.184. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27829901 Free PMC article. Review.
Cited by
-
Ynamide carbopalladation: a flexible route to mono-, bi- and tricyclic azacycles.Chemistry. 2015 Sep 1;21(36):12627-39. doi: 10.1002/chem.201501710. Epub 2015 Jul 16. Chemistry. 2015. PMID: 26189754 Free PMC article.
-
Selective molecular sequestration with concurrent natural product functionalization and derivatization: from crude natural product extracts to a single natural product derivative in one step.J Org Chem. 2011 Dec 16;76(24):10249-53. doi: 10.1021/jo201361s. Epub 2011 Nov 16. J Org Chem. 2011. PMID: 22059469 Free PMC article.
-
QTAIM and IRC studies for the evaluation of activation energy on the C=P, C=N and C=O Diels-Alder reaction.Heliyon. 2020 Aug 19;6(8):e04655. doi: 10.1016/j.heliyon.2020.e04655. eCollection 2020 Aug. Heliyon. 2020. PMID: 32904344 Free PMC article.
-
Origins of regio- and stereochemistry in type 2 intramolecular N-acylnitroso Diels-Alder reactions: a computational study of tether length and substituent effects.J Org Chem. 2013 Apr 19;78(8):4090-8. doi: 10.1021/jo4004025. Epub 2013 Mar 11. J Org Chem. 2013. PMID: 23477601 Free PMC article.
-
Diastereoselective Three-Component 3,4-Amino Oxygenation of 1,3-Dienes Catalyzed by a Cationic Heptamethylindenyl Rhodium(III) Complex.J Am Chem Soc. 2021 Nov 3;143(43):17964-17969. doi: 10.1021/jacs.1c09276. Epub 2021 Oct 20. J Am Chem Soc. 2021. PMID: 34668705 Free PMC article.
References
-
- Streith J, Defoin A. Synthesis. 1994:1107.
-
- Weinreb SM, Staib RR. Tetrahedron. 1982;38:3087.
-
- Streith J, Defoin A. Synlett. 1996:189.
-
- Vogt PF, Miller MJ. Tetrahedron. 1998;54:1317.
-
- Yamamoto Y, Yamamoto H. Eur J Org Chem. 2006:2031.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

