Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction
- PMID: 21521247
- PMCID: PMC3229680
- DOI: 10.1111/j.1574-6976.2011.00276.x
Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction
Abstract
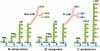
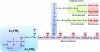
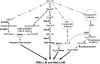
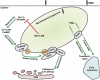
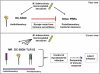
Approximately one third of the world's population is infected with Mycobacterium tuberculosis, the causative agent of tuberculosis. This bacterium has an unusual lipid-rich cell wall containing a vast repertoire of antigens, providing a hydrophobic impermeable barrier against chemical drugs, thus representing an attractive target for vaccine and drug development. Apart from the mycolyl-arabinogalactan-peptidoglycan complex, mycobacteria possess several immunomodulatory constituents, notably lipomannan and lipoarabinomannan. The availability of whole-genome sequences of M. tuberculosis and related bacilli over the past decade has led to the identification and functional characterization of various enzymes and the potential drug targets involved in the biosynthesis of these glycoconjugates. Both lipomannan and lipoarabinomannan possess highly variable chemical structures, which interact with different receptors of the immune system during host-pathogen interactions, such as Toll-like receptors-2 and C-type lectins. Recently, the availability of mutants defective in the synthesis of these glycoconjugates in mycobacteria and the closely related bacterium, Corynebacterium glutamicum, has paved the way for host-pathogen interaction studies, as well as, providing attenuated strains of mycobacteria for the development of new vaccine candidates. This review provides a comprehensive account of the structure, biosynthesis and immunomodulatory properties of these important glycoconjugates.
© 2011 Federation of European Microbiological Societies. Published by Blackwell Publishing Ltd. All rights reserved.
Figures

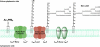
References
-
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Alderwick LJ, Radmacher E, Seidel M, Gande R, Hitchen PG, Morris HR, Dell A, Sahm H, Eggeling L, Besra GS. Deletion of Cg-emb in Corynebacterineae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J Biol Chem. 2005;280:32362–32371. - PubMed
-
- Alderwick LJ, Dover LG, Seidel M, Gande R, Sahm H, Eggeling L, Besra GS. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-d-arabinose metabolism. Glycobiology. 2006a;16:1073–1081. - PubMed
-
- Alderwick LJ, Seidel M, Sahm H, Besra GS, Eggeling L. Identification of a novel arabinofuranosyltransferase (AftA) involved in cell wall arabinan biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2006c;281:15653–15661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

