Point-of-care oral-based diagnostics
- PMID: 21521419
- PMCID: PMC4273652
- DOI: 10.1111/j.1601-0825.2011.01808.x
Point-of-care oral-based diagnostics
Abstract
Many of the target molecules that reside in blood are also present in oral fluids, albeit at lower concentrations. Oral fluids are, however, relatively easy and safe to collect without the need for specialized equipment and training. Thus, oral fluids provide convenient samples for medical diagnostics. Recent advances in lab-on-a-chip technologies have made minute, fully integrated diagnostic systems practical for an assortment of point-of-care tests. Such systems can perform either immunoassays or molecular diagnostics outside centralized laboratories within time periods ranging from minutes to an hour. The article briefly reviews recent advances in devices for point-of-care testing with a focus on work that has been carried out by the authors as part of a NIH program.
© 2011 John Wiley & Sons A/S.
Figures
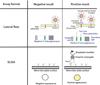

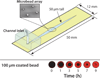

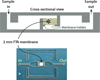
References
-
- Ali MF, Kirby R, Goodey AP, et al. DNA hybridization and discrimination of single-nucleotide mismatches using chip-based microbead arrays. Anal Chem. 2003;75:4732–4739. - PubMed
-
- Ashihara Y, Kasahara Y, Nakamura R. Immunoassay and immunochemistry. Chapter 43. In: McPherson RA, Pincus MR, editors. Henry’s clinical diagnosis and management by laboratory methods. Philadelphia, PA, USA: Saunders Elsevier; 2007. pp. 793–818.
-
- Birkhahn RH, Haines E, Wen W, Reddy L, Briggs WM, Datillo PA. Estimating the clinical impact of bringing a multimarker cardiac panel to the bedside in the ED. [accessed on 5 January 2011];Am J Emerg Med. 2010 Available at: http://www.ncbi.nlm.nih.gov/pubmed/20825823. - PubMed
-
- Bowden M, Song L, Walt DR. Development of a microfluidic platform with an optical imaging microarray capable of attomolar target DNA detection. Anal Chem. 2005;77:5583–5588. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

