CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates
- PMID: 21521467
- PMCID: PMC4845096
- DOI: 10.1111/j.1600-6143.2011.03509.x
CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates
Abstract
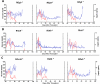
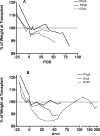
The widespread clinical implementation of alloislet transplantation as therapy for type 1 diabetes has been hindered by the lack of suitable islet donors. Pig-to-human islet xenotransplantation is one strategy with potential to alleviate this shortage. Long-term survival of porcine islets has been achieved using CD154-specific antibodies to interrupt the CD40/CD154 costimulation pathway; however, CD154-specific antibodies seem unlikely candidates for clinical translation. An alternative strategy for CD40/CD154 pathway interruption is use of CD40-specific antibodies. Herein, we evaluate the ability of a chimeric CD40-specific monoclonal antibody (Chi220) to protect islet xenografts. Neonatal porcine islets (~50,000 IEQ/kg) were transplanted intraportally into pancreatectomized diabetic macaques. Immunosuppression consisted of induction therapy with Chi220 and the IL-2 receptor-specific antibody basiliximab, and maintenance therapy with sirolimus and the B7-specific fusion protein belatacept. Chi220 effectively promoted xenoislet engraftment and survival, with five of six treated recipients achieving insulin-independent normoglycemia (median rejection-free survival 59 days; mean 90.8 days, maximum 203 days). No thromboembolic phenomena were observed. CD40 represents a promising alternative to CD154 as a therapeutic target, and the efficacy of CD40-specific antibodies in islet xenotransplantation warrants further investigation.
©2011 The Authors Journal compilation©2011 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures




References
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. - PubMed
-
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. - PubMed
-
- Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol. 2009;21(2):81–86. - PubMed
-
- Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7(7):519–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

