The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits
- PMID: 21521608
- PMCID: PMC3119519
- DOI: 10.1016/j.neuron.2011.04.007
The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits
Abstract
Ionotropic glutamate receptors (iGluRs) underlie rapid, excitatory synaptic signaling throughout the CNS. After years of intense research, our picture of iGluRs has evolved from them being companionless in the postsynaptic membrane to them being the hub of dynamic supramolecular signaling complexes, interacting with an ever-expanding litany of other proteins that regulate their trafficking, scaffolding, stability, signaling, and turnover. In particular, the discovery that transmembrane AMPA receptor regulatory proteins (TARPs) are AMPA receptor auxiliary subunits that are critical determinants of their trafficking, gating, and pharmacology has changed the way we think about iGluR function. Recently, a number of novel transmembrane proteins have been uncovered that may also serve as iGluR auxiliary proteins. Here we review pivotal developments in our understanding of the role of TARPs in AMPA receptor trafficking and gating, and provide an overview of how newly discovered transmembrane proteins expand our view of iGluR function in the CNS.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

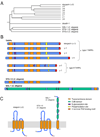


References
-
- Abouda H, Hizem Y, Gargouri A, Depienne C, Bouteiller D, Riant F, Tournier-Lasserve E, Gourfinkel-An I, LeGuern E, Gouider R. Familial form of typical childhood absence epilepsy in a consanguineous context. Epilepsia. 2010;51:1889–1893. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. - PubMed
-
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

