Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation
- PMID: 21521698
- PMCID: PMC3101552
- DOI: 10.1105/tpc.110.081067
Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation
Abstract
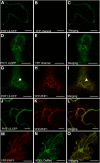
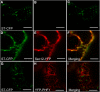
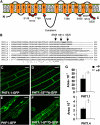
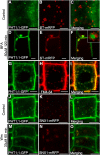
In Arabidopsis thaliana, the PHOSPHATE TRANSPORTER1 (PHT1) family encodes the high-affinity phosphate transporters. They are transcriptionally induced by phosphate starvation and require PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR (PHF1) to exit the endoplasmic reticulum (ER), indicating intracellular traffic as an additional level of regulation of PHT1 activity. Our study revealed that PHF1 acts on PHT1, upstream of vesicle coat protein COPII formation, and that additional regulatory events occur during PHT1 trafficking and determine its ER exit and plasma membrane stability. Phosphoproteomic and mutagenesis analyses revealed modulation of PHT1;1 ER export by Ser-514 phosphorylation status. Confocal microscopy analysis of root tip cells showed that PHT1;1 is localized to the plasma membrane and is present in intracellular endocytic compartments. More precisely, PHT1;1 was localized to sorting endosomes associated with prevacuolar compartments. Kinetic analysis of PHT1;1 stability and targeting suggested a modulation of PHT1 internalization from the plasma membrane to the endosomes, followed by either subsequent recycling (in low Pi) or vacuolar degradation (in high Pi). For the latter condition, we identified a rapid mechanism that reduces the pool of PHT1 proteins present at the plasma membrane. This mechanism is regulated by the Pi concentration in the medium and appears to be independent of degradation mechanisms potentially regulated by the PHO2 ubiquitin conjugase. We propose a model for differential trafficking of PHT1 to the plasma membrane or vacuole as a function of phosphate concentration.
Figures



References
-
- Barber S.A., Walker J.M., Vasey E.H. (1963). Mechanisms for movement of plant nutrients from soil and fertilizer to plant root. J. Agric. Food Chem. 11: 204–207
-
- Bolte S., Cordelières F.P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224: 213–232 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

