Direct reprogramming of mouse fibroblasts to neural progenitors
- PMID: 21521790
- PMCID: PMC3093517
- DOI: 10.1073/pnas.1103113108
Direct reprogramming of mouse fibroblasts to neural progenitors
Abstract
The simple yet powerful technique of induced pluripotency may eventually supply a wide range of differentiated cells for cell therapy and drug development. However, making the appropriate cells via induced pluripotent stem cells (iPSCs) requires reprogramming of somatic cells and subsequent redifferentiation. Given how arduous and lengthy this process can be, we sought to determine whether it might be possible to convert somatic cells into lineage-specific stem/progenitor cells of another germ layer in one step, bypassing the intermediate pluripotent stage. Here we show that transient induction of the four reprogramming factors (Oct4, Sox2, Klf4, and c-Myc) can efficiently transdifferentiate fibroblasts into functional neural stem/progenitor cells (NPCs) with appropriate signaling inputs. Compared with induced neurons (or iN cells, which are directly converted from fibroblasts), transdifferentiated NPCs have the distinct advantage of being expandable in vitro and retaining the ability to give rise to multiple neuronal subtypes and glial cells. Our results provide a unique paradigm for iPSC-factor-based reprogramming by demonstrating that it can be readily modified to serve as a general platform for transdifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
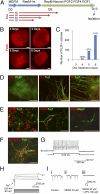
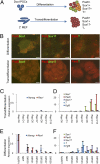


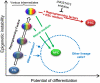
References
-
- Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

