Study of the biological activity of novel synthetic compounds with antiviral properties against human rhinoviruses
- PMID: 21522081
- PMCID: PMC6263271
- DOI: 10.3390/molecules16053479
Study of the biological activity of novel synthetic compounds with antiviral properties against human rhinoviruses
Abstract
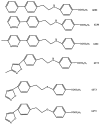
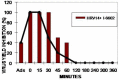
Picornaviridae represent a very large family of small RNA viruses, some of which are the cause of important human and animal diseases. Since no specific therapy against any of these viruses currently exists, palliative symptomatic treatments are employed. The early steps of the picornavirus replicative cycle seem to be privileged targets for some antiviral compounds like disoxaril and pirodavir. Pirodavir's main weakness is its cytotoxicity on cell cultures at relatively low doses. In this work some original synthetic compounds were tested, in order to find less toxic compounds with an improved protection index (PI) on infected cells. Using an amino group to substitute the oxygen atom in the central chain, such as that in the control molecule pirodavir, resulted in decreased activity against Rhinoviruses and Polioviruses. The presence of an -ethoxy-propoxy- group in the central chain (as in compound I-6602) resulted in decreased cell toxicity and in improved anti-Rhinovirus activity. This compound actually showed a PI >700 on HRV14, while pirodavir had a PI of 250. These results demonstrate that modification of pirodavir's central hydrocarbon chain can lead to the production of novel derivatives with low cytotoxicity and improved PI against some strains of Rhinoviruses.
Figures

References
-
- Rueckert R.R. Picornaviruses and their replication. In: Channock R.M., Hirsch M.S., Melnick J.L., Monath T.P., Roizman B., editors. Fields Virology. 2nd ed. Raven Press; New York, NY, USA: 1990. pp. 507–548.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

