The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein
- PMID: 21522185
- PMCID: PMC3179363
- DOI: 10.1038/ejhg.2011.63
The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein
Abstract
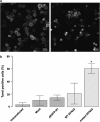
DFNA5 was first identified as a gene causing autosomal dominant hearing loss (HL). Different mutations have been found, all exerting a highly specific gain-of-function effect, in which skipping of exon 8 causes the HL. Later reports revealed the involvement of the gene in different types of cancer. Epigenetic silencing of DFNA5 in a large percentage of gastric, colorectal and breast tumors and p53-dependent transcriptional activity have been reported, concluding that DFNA5 acts as a tumor suppressor gene in different frequent types of cancer. Despite these data, the molecular function of DFNA5 has not been investigated properly. Previous transfection studies with mutant DFNA5 in yeast and in mammalian cells showed a toxic effect of the mutant protein, which was not seen after transfection of the wild-type protein. Here, we demonstrate that DFNA5 is composed of two domains, separated by a hinge region. The first region induces apoptosis when transfected in HEK293T cells, the second region masks and probably regulates this apoptosis inducing capability. Moreover, the involvement of DFNA5 in apoptosis-related pathways in a physiological setting was demonstrated through gene expression microarray analysis using Dfna5 knockout mice. In view of its important role in carcinogenesis, this finding is expected to lead to new insights on the role of apoptosis in many types of cancer. In addition, it provides a new line of evidence supporting an important role for apoptosis in monogenic and complex forms of HL.
Figures



References
-
- Van Laer L, Huizing EH, Verstreken M, et al. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20:194–197. - PubMed
-
- Cheng J, Han DY, Dai P, et al. A novel DFNA5 mutation, IVS8+4 A>G, in the splice donor site of intron 8 causes late-onset non-syndromic hearing loss in a Chinese family. Clin Genet. 2007;72:471–477. - PubMed
-
- Bischoff AMLC, Luijendijk MWJ, Huygen PLM, et al. A second mutation identified in the DFNA5 gene in a Dutch family. A clinical and genetic evaluation. Audiol Neuro-otol. 2004;9:34–36. - PubMed
-
- Yu C, Meng X, Zhang S, Zhao G, Hu L, Kong X. A 3-nucleotide deletion in the polypyrimidine tract of intron 7 of the DFNA5 gene causes nonsyndromic hearing impairment in a Chinese family. Genomics. 2003;82:575–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

