Integrated carbon fiber electrodes within hollow polymer microneedles for transdermal electrochemical sensing
- PMID: 21522504
- PMCID: PMC3082351
- DOI: 10.1063/1.3569945
Integrated carbon fiber electrodes within hollow polymer microneedles for transdermal electrochemical sensing
Abstract
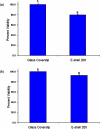
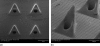
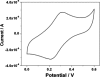
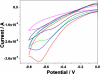
In this study, carbon fiber electrodes were incorporated within a hollow microneedle array, which was fabricated using a digital micromirror device-based stereolithography instrument. Cell proliferation on the acrylate-based polymer used in microneedle fabrication was examined with human dermal fibroblasts and neonatal human epidermal keratinocytes. Studies involving full-thickness cadaveric porcine skin and trypan blue dye demonstrated that the hollow microneedles remained intact after puncturing the outermost layer of cadaveric porcine skin. The carbon fibers underwent chemical modification in order to enable detection of hydrogen peroxide and ascorbic acid; electrochemical measurements were demonstrated using integrated electrode-hollow microneedle devices.
Figures





Similar articles
-
Hollow microneedle-based sensor for multiplexed transdermal electrochemical sensing.J Vis Exp. 2012 Jun 1;(64):e4067. doi: 10.3791/4067. J Vis Exp. 2012. PMID: 22688693 Free PMC article.
-
The Effects of Geometry on Skin Penetration and Failure of Polymer Microneedles.J Adhes Sci Technol. 2013 Feb 1;27(3):227-243. doi: 10.1080/01694243.2012.705101. Epub 2012 Aug 6. J Adhes Sci Technol. 2013. PMID: 23543070 Free PMC article.
-
Microneedle array-based carbon paste amperometric sensors and biosensors.Analyst. 2011 May 7;136(9):1846-51. doi: 10.1039/c1an00012h. Epub 2011 Mar 16. Analyst. 2011. PMID: 21412519
-
Current trends in polymer microneedle for transdermal drug delivery.Int J Pharm. 2020 Sep 25;587:119673. doi: 10.1016/j.ijpharm.2020.119673. Epub 2020 Jul 30. Int J Pharm. 2020. PMID: 32739388 Free PMC article. Review.
-
Research progress of advanced microneedle drug delivery system and its application in biomedicine.Colloids Surf B Biointerfaces. 2023 Jun;226:113302. doi: 10.1016/j.colsurfb.2023.113302. Epub 2023 Apr 7. Colloids Surf B Biointerfaces. 2023. PMID: 37086686 Review.
Cited by
-
Flow biosensing and sampling in indirect electrochemical detection.Biomicrofluidics. 2012 Jun;6(2):24114-2411413. doi: 10.1063/1.4705368. Epub 2012 Apr 20. Biomicrofluidics. 2012. PMID: 22655022 Free PMC article.
-
Echem methods and electrode types of the current in vivo electrochemical sensing.RSC Adv. 2022 Jun 15;12(28):17715-17739. doi: 10.1039/d2ra01273a. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35765338 Free PMC article. Review.
-
Customizable Fabrication of Photothermal Microneedles with Plasmonic Nanoparticles Using Low-Cost Stereolithography Three-Dimensional Printing.ACS Appl Bio Mater. 2024 Jul 15;7(7):4533-4541. doi: 10.1021/acsabm.4c00411. Epub 2024 Jun 15. ACS Appl Bio Mater. 2024. PMID: 38877987 Free PMC article.
-
Microneedles for drug and vaccine delivery.Adv Drug Deliv Rev. 2012 Nov;64(14):1547-68. doi: 10.1016/j.addr.2012.04.005. Epub 2012 May 1. Adv Drug Deliv Rev. 2012. PMID: 22575858 Free PMC article. Review.
-
Optimizing bioconversion of ferulic acid to vanillin by Bacillus subtilis in the stirred packed reactor using Box-Behnken design and desirability function.Food Sci Biotechnol. 2017 Feb 28;26(1):143-152. doi: 10.1007/s10068-017-0019-0. eCollection 2017. Food Sci Biotechnol. 2017. PMID: 30263521 Free PMC article.
References
-
- Justino C. I. L., Rocha-Santos T. A., and Duarte A. C., Trends Analyt. Chem. ZZZZZZ 29, 1172 (2010).10.1016/j.trac.2010.07.008 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
