2,6-Bis(2-methyl-1,3-diazinan-2-yl)pyridine
- PMID: 21522750
- PMCID: PMC3050144
- DOI: 10.1107/S1600536810050063
2,6-Bis(2-methyl-1,3-diazinan-2-yl)pyridine
Abstract
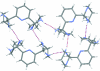
The title compound, C(15)H(25)N(5), is an aminalization product between 2,6-diacetyl-pyridine and 1,3-diamino-propane. It crystallizes with two independent mol-ecules in the asymmetric unit with different conformations. In the first mol-ecule, the methyl groups are cis oriented with respect to the pyridine ring [N-C-C-C torsion angles = 72.5 (1) and 80.3 (1)°], while they are trans oriented in the second mol-ecule [N-C-C-C torsion angles = 82.6 (1) and -90.8 (1)°]. Each of the two mol-ecules forms centrosymmetric dimers held together by N-H⋯N hydrogen bonds, thus forming R(2) (2)(16) rings. The two dimers are inter-linked by additional N-H⋯N bonds into R(4) (4)(14) rings, building chains along the a axis. These patterns influence the orientation (either equatorial or axial) of the N-H bonds.
Figures
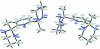
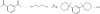
References
-
- Burnet, S., Hall, A. K., Hall, J. M., Harrowfield, J. M., Koutsantonis, G. A., Sanford, V., Sauter, D., Skelton, B. W. & White, A. H. (2003). Supramol. Chem. 15, 291–312.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
-
- Stoe & Cie (2001). X-AREA Stoe & Cie, Darmstadt, Germany.
-
- Thalladi, V. R., Boese, R. & Weiss, C.-H. (2000). Angew. Chem. Int. Ed. 39, 918–922. - PubMed
-
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
LinkOut - more resources
Full Text Sources
Miscellaneous
