1-Dibenzylamino-1-de-oxy-4,5-O-isopropyl-idene-β-d-fructopyran-ose
- PMID: 21523062
- PMCID: PMC3051515
- DOI: 10.1107/S1600536810053973
1-Dibenzylamino-1-de-oxy-4,5-O-isopropyl-idene-β-d-fructopyran-ose
Abstract
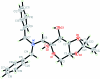
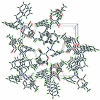
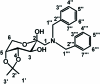
The title compound C(23)H(29)NO(5), synthesized by the Amadori rearrangement of α-d-glucose with dibenzyl-amine and the ketalization, is shown to be a β-anomer. The fructopyran-ose ring adopts a chair conformation. The two benzene rings form a dihedral angle of 68.9 (1)°. In the crystal, non-classical inter-molecular C-H⋯O hydrogen bonds link the mol-ecules into a three-dimensional network.
Figures

References
-
- Bruker (2001). SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2005). SMART Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
-
- Shu, L., Shen, Y.-M., Burke, C., Goeddel, D. & Shi, Y. (2003). J. Org. Chem. 68, 4963–4965. - PubMed
-
- Tian, H., She, X., Yu, H., Shu, L. & Shi, Y. (2002). J. Org. Chem. 67, 2435–2446. - PubMed
LinkOut - more resources
Full Text Sources
