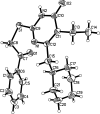
6-Cyclo-hexyl-meth-yl-5-ethyl-2-[(2-oxo-2-phenyl-eth-yl)sulfan-yl]pyrimidin-4(3H)-one
- PMID: 21523182
- PMCID: PMC3051611
- DOI: 10.1107/S1600536811003175
6-Cyclo-hexyl-meth-yl-5-ethyl-2-[(2-oxo-2-phenyl-eth-yl)sulfan-yl]pyrimidin-4(3H)-one
Abstract
In the title compound, C(21)H(26)N(2)O(2)S, the cyclo-hexane ring adopts a chair conformation. The angle at the methyl-ene bridge linking the pyrimidine and cyclo-hexane rings is 113.41 (13)°. This is in the range considered optimal for maximum activity of non-nucleoside reverse transcriptase inhibitors. In the crystal, mol-ecules are connected into centrosymmetric dimers via pairs of N-H⋯O hydrogen bonds.
Figures
References
-
- Bruker (2004). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Ettorre, A., Mai, A., Artico, M., Massa, S., De Montis, A. & La Colla, P. (1996). Acta Cryst. C52, 2115–2117.
-
- Ettorre, A., Mai, A., Sbardella, G., Artico, M., La Colla, P. & Massa, S. (1998). Z. Kristallogr. New Cryst. Struct. 213, 593–595.
-
- He, Y. P., Long, J., Zhang, S. S., Li, C., Lai, C., Zhang, C. S., Li, D. X., Zhang, D. H., Wang, H., Cai, Q. Q. & Zheng, Y. T. (2011). Bioorg. Med. Chem. Lett. 21, 694–697. - PubMed
-
- Rao, Z.-K., Zhang, S.-S., He, Y.-P., Zheng, Y.-T. & Li, C. (2007). Acta Cryst. E63, o3942.
LinkOut - more resources
Full Text Sources