Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria
- PMID: 21524089
- PMCID: PMC3133741
- DOI: 10.1021/jm101621u
Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria
Abstract
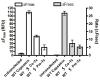
New compounds nobilamides A-H and related known compounds A-3302-A and A-3302-B were isolated based upon their suppression of capsaicin-induced calcium uptake in a mouse dorsal root ganglion primary cell culture assay. Two of these compounds, nobilamide B and A-3302-A, were shown to be long-acting antagonists of mouse and human TRPV1 channels, abolishing activity for >1 h after removal of drug presumably via a covalent attachment. Other derivatives also inhibited the TRPV1 channel, albeit with low potency, affording a structure-activity profile to support the proposed mechanism of action. While the activities were modest, we propose a new mechanism of action and a new site of binding for these inhibitors that may spur development of related analogues for treatment of pain.
Figures

References
-
- Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radical Bio Med. 2007;43:1670. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Szallasi A, Appendino G. Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. J Med Chem. 2004;47:2717–2723. - PubMed
-
- Malmberg AB, Bley KR, editors. Progress in Inflammation Research. Birkhäuser-Verlag; Boston: 2005. Turning up the Heat on Pain: TRPV1 Receptors in Pain and Inflammation.
-
- Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14:56–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

