Spinal associative stimulation: a non-invasive stimulation paradigm to modulate spinal excitability
- PMID: 21524606
- PMCID: PMC3538079
- DOI: 10.1016/j.clinph.2011.02.038
Spinal associative stimulation: a non-invasive stimulation paradigm to modulate spinal excitability
Abstract
Objective: Repetitive, paired peripheral and transcranial stimulation targeting the cerebral cortex can increase cortical excitability, outlasting the stimulation period. It is unknown whether paired stimulation specifically targeting the spinal cord can modulate spinal excitability. We tested whether the H-reflex facilitation from a sub-threshold conditioning TMS pulse could modulate spinal excitability if delivered repetitively.
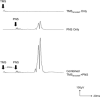
Method: In 13 healthy subjects, we delivered single-pulse TMS (80% RMT) for the right soleus muscle, 20 ms prior to an electrical peripheral nerve stimulus delivered over the posterior tibial nerve on the same side at 0.1 Hz during 15 min.
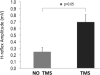
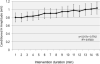
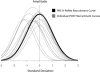
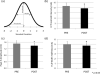
Results: PNS alone evoked an H-reflex of 0.25 mV ± 0.06 SEM, while pairing of TMS and PNS facilitated the H-reflex to 0.7 ± 0.11 mV. TMS-PNS pairs delivered at 0.1 Hz for 15 min progressively increased in the evoked response to ∼130% (r(2) = 0.97) of the starting amplitude (normalized to 1st min). Post-intervention, H-reflex threshold decreased (pre = 12.9 ± 1.7 mA; post =11.6 ± 1.6 mA; p = 0.04), as did the stimulus intensity at maximum H-reflex amplitude (pre = 23.5 ± 02.8 mA; post = 21.6 ± 2.6 mA; p = 0.03), and recruitment curve width (pre = 11.6 ± 1.5 mA; post = 10.93 ± 1.4 mA; p = 0.03). No such changes were observed with intervention of PNS or TMS alone.
Conclusion: Paired stimulation targeting spinal facilitatory interactions, when applied repetitively, can increase spinal excitability during and after the intervention.
Significance: Spinal associative stimulation may have potential for neuromodulation in spinal cord injury patients.
Copyright © 2011 International Federation of Clinical Neurophysiology. Published by Elsevier Ireland Ltd. All rights reserved.
Figures



Comment in
-
Non-invasive tools to promote spinal plasticity in humans.Clin Neurophysiol. 2011 Nov;122(11):2114-5. doi: 10.1016/j.clinph.2011.03.024. Epub 2011 Apr 22. Clin Neurophysiol. 2011. PMID: 21514881 No abstract available.
References
-
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. - PubMed
-
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
-
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

