Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles
- PMID: 21525001
- PMCID: PMC3122239
- DOI: 10.1074/jbc.M111.238519
Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles
Abstract
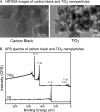
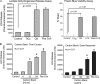
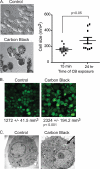
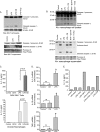
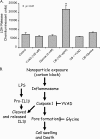
Inhalation of nanoparticles has been implicated in respiratory morbidity and mortality. In particular, carbon black nanoparticles are found in many different environmental exposures. Macrophages take up inhaled nanoparticles and respond via release of inflammatory mediators and in some cases cell death. Based on new data, we propose that exposure of macrophages (both a macrophage cell line and primary human alveolar macrophages) to carbon black nanoparticles induces pyroptosis, an inflammasome-dependent form of cell death. Exposure of macrophages to carbon black nanoparticles resulted in inflammasome activation as defined by cleavage of caspase 1 to its active form and downstream IL-1β release. The cell death that occurred with carbon black nanoparticle exposure was identified as pyroptosis by the protective effect of a caspase 1 inhibitor and a pyroptosis inhibitor. These data demonstrate that carbon black nanoparticle exposure activates caspase 1, increases IL-1β release after LPS priming, and induces the proinflammatory cell death, pyroptosis. The identification of pyroptosis as a cellular response to carbon nanoparticle exposure is novel and relates to environmental and health impacts of carbon-based particulates.
Figures
Comment in
-
Comments on induction of inflammasome-dependent pyroptosis by carbon black nanoparticles.J Biol Chem. 2011 Sep 23;286(38):le17; author reply le18. doi: 10.1074/jbc.L111.238519. J Biol Chem. 2011. PMID: 21926176 Free PMC article. No abstract available.
References
-
- Opitz B., van Laak V., Eitel J., Suttorp N. (2010) Am. J. Respir. Crit. Care Med. 181, 1294–1309 - PubMed
-
- Harada R. N., Repine J. E. (1985) Chest 87, 247–252 - PubMed
-
- Twigg H. L., 3rd (2004) Semin. Respir. Crit. Care Med. 25, 21–31 - PubMed
-
- Sibille Y., Reynolds H. Y. (1990) Am. Rev. Respir. Dis. 141, 471–501 - PubMed
-
- Loeffler J., Hedderich R., Fiedeler U., Malsch I., Túquerres G., Koskinen J., Linden M., Lojkowski W., Moritz T., Zins M., Bernabeu E., Larena A. (2010) in Nanoroad SME European Project, European Union, Brussels, Belgium
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

