C4orf41 and TTC-15 are mammalian TRAPP components with a role at an early stage in ER-to-Golgi trafficking
- PMID: 21525244
- PMCID: PMC3113772
- DOI: 10.1091/mbc.E10-11-0873
C4orf41 and TTC-15 are mammalian TRAPP components with a role at an early stage in ER-to-Golgi trafficking
Abstract
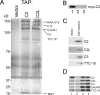
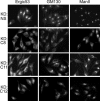
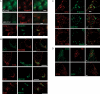
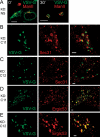
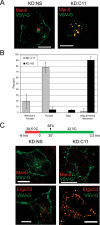
TRAPP is a multisubunit tethering complex implicated in multiple vesicle trafficking steps in Saccharomyces cerevisiae and conserved throughout eukarya, including humans. Here we confirm the role of TRAPPC2L as a stable component of mammalian TRAPP and report the identification of four novel components of the complex: C4orf41, TTC-15, KIAA1012, and Bet3L. Two of the components, KIAA1012 and Bet3L, are mammalian homologues of Trs85p and Bet3p, respectively. The remaining two novel TRAPP components, C4orf41 and TTC-15, have no homologues in S. cerevisiae. With this work, human homologues of all the S. cerevisiae TRAPP proteins, with the exception of the Saccharomycotina-specific subunit Trs65p, have now been reported. Through a multidisciplinary approach, we demonstrate that the novel proteins are bona fide components of human TRAPP and implicate C4orf41 and TTC-15 (which we call TRAPPC11 and TRAPPC12, respectively) in ER-to-Golgi trafficking at a very early stage. We further present a binary interaction map for all known mammalian TRAPP components and evidence that TRAPP oligomerizes. Our data are consistent with the absence of a TRAPP I-equivalent complex in mammalian cells, suggesting that the fundamental unit of mammalian TRAPP is distinct from that characterized in S. cerevisiae.
Figures

References
-
- Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–448. - PubMed
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

