Cross-linking by protein oxidation in the rapidly setting gel-based glues of slugs
- PMID: 21525316
- PMCID: PMC3083248
- DOI: 10.1242/jeb.051581
Cross-linking by protein oxidation in the rapidly setting gel-based glues of slugs
Abstract
The terrestrial slug Arion subfuscus secretes a glue that is a dilute gel with remarkable adhesive and cohesive strength. The function of this glue depends on metals, raising the possibility that metal-catalyzed oxidation plays a role. The extent and time course of protein oxidation was measured by immunoblotting to detect the resulting carbonyl groups. Several proteins, particularly one with a relative molecular mass (M(r)) of 165 x 10³, were heavily oxidized. Of the proteins known to distinguish the glue from non-adhesive mucus, only specific size variants were oxidized. The oxidation appears to occur within the first few seconds of secretion. Although carbonyls were detected by 2,4-dinitrophenylhydrazine (DNPH) in denatured proteins, they were not easily detected in the native state. The presence of reversible cross-links derived from carbonyls was tested for by treatment with sodium borohydride, which would reduce uncross-linked carbonyls to alcohols, but stabilize imine bonds formed by carbonyls and thus lead to less soluble complexes. Consistent with imine bond formation, sodium borohydride led to a 20-35% decrease in the amount of soluble protein with a M(r) of 40-165 (x 10³) without changing the carbonyl content per protein. In contrast, the nucleophile hydroxylamine, which would competitively disrupt imine bonds, increased protein solubility in the glue. Finally, the primary amine groups on a protein with a M(r) of 15 x 10³ were not accessible to acid anhydrides. The results suggest that cross-links between aldehydes and primary amines contribute to the cohesive strength of the glue.
Figures
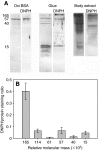

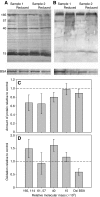
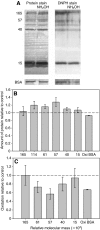

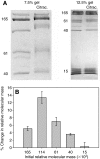
References
-
- Berlett B. S., Stadtman E. R. (1997). Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272, 20313-20316 - PubMed
-
- Chen T., Embree H. D., Brown E. M., Taylor M. M., Payne G. F. (2003). Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications. Biomaterials 24, 2831-2841 - PubMed
-
- Coyne K. J., Qin X. X., Waite J. H. (1997). Extensible collagen in mussel byssus: a natural block copolymer. Science 277, 1830-1832 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

