Clathrin-mediated endocytosis in living host cells visualized through quantum dot labeling of infectious hematopoietic necrosis virus
- PMID: 21525360
- PMCID: PMC3126507
- DOI: 10.1128/JVI.00109-11
Clathrin-mediated endocytosis in living host cells visualized through quantum dot labeling of infectious hematopoietic necrosis virus
Abstract
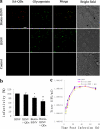
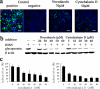
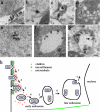
Infectious hematopoietic necrosis virus (IHNV) is an important fish pathogen that infects both wild and cultured salmonids. As a species of the genus Novirhabdovirus, IHNV is a valuable model system for exploring the host entry mechanisms of rhabdoviruses. In this study, quantum dots (QDs) were used as fluorescent labels for sensitive, long-term tracking of IHNV entry. Using live-cell fluorescence microscopy, we found that IHNV is internalized through clathrin-coated pits after the virus binds to host cell membranes. Pretreatment of host cells with chlorpromazine, a drug that blocks clathrin-mediated endocytosis, and clathrin light chain (LCa) depletion using RNA interference both resulted in a marked reduction in viral entry. We also visualized transport of the virus via the cytoskeleton (i.e., actin filaments and microtubules) in real time. Actin polymerization is involved in the transport of endocytic vesicles into the cytosol, whereas microtubules are required for the trafficking of clathrin-coated vesicles to early endosomes, late endosomes, and lysosomes. Disrupting the host cell cytoskeleton with cytochalasin D or nocodazole significantly impaired IHNV infectivity. Furthermore, infection was significantly affected by pretreating the host cells with bafilomycin A1, a compound that inhibits the acidification of endosomes and lysosomes. Strong colocalizations of IHNV with endosomes indicated that the virus is internalized into these membrane-bound compartments. This is the first report in which QD labeling is used to visualize the dynamic interactions between viruses and endocytic structures; the results presented demonstrate that IHNV enters host cells via clathrin-mediated endocytic, cytoskeleton-dependent, and low-pH-dependent pathways.
Figures

Similar articles
-
Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway.J Virol. 2004 Oct;78(19):10543-55. doi: 10.1128/JVI.78.19.10543-10555.2004. J Virol. 2004. PMID: 15367621 Free PMC article.
-
Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells.PLoS Pathog. 2009 Jul;5(7):e1000512. doi: 10.1371/journal.ppat.1000512. Epub 2009 Jul 10. PLoS Pathog. 2009. PMID: 19593382 Free PMC article.
-
Single-Particle Tracking Reveals the Sequential Entry Process of the Bunyavirus Severe Fever with Thrombocytopenia Syndrome Virus.Small. 2019 Feb;15(6):e1803788. doi: 10.1002/smll.201803788. Epub 2018 Dec 27. Small. 2019. PMID: 30589216
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
-
Insider information: what viruses tell us about endocytosis.Curr Opin Cell Biol. 2003 Aug;15(4):414-22. doi: 10.1016/s0955-0674(03)00081-4. Curr Opin Cell Biol. 2003. PMID: 12892781 Review.
Cited by
-
Current Advances in the Biomedical Applications of Quantum Dots: Promises and Challenges.Int J Mol Sci. 2023 Aug 11;24(16):12682. doi: 10.3390/ijms241612682. Int J Mol Sci. 2023. PMID: 37628860 Free PMC article. Review.
-
Entry of Challenge Virus Standard (CVS) -11 into N2a cells via a clathrin-mediated, cholesterol-, dynamin-, pH-dependent endocytic pathway.Virol J. 2019 Jun 13;16(1):80. doi: 10.1186/s12985-019-1186-9. Virol J. 2019. PMID: 31196105 Free PMC article.
-
Cross Talk between Viruses and Insect Cells Cytoskeleton.Viruses. 2021 Aug 20;13(8):1658. doi: 10.3390/v13081658. Viruses. 2021. PMID: 34452522 Free PMC article. Review.
-
Tracking single baculovirus retrograde transportation in host cell via quantum dot-labeling of virus internal component.J Nanobiotechnology. 2017 May 6;15(1):37. doi: 10.1186/s12951-017-0270-9. J Nanobiotechnology. 2017. PMID: 28477617 Free PMC article.
-
Kinetics of cellular uptake of viruses and nanoparticles via clathrin-mediated endocytosis.Phys Biol. 2016 Feb 12;13(1):016005. doi: 10.1088/1478-3975/13/1/016005. Phys Biol. 2016. PMID: 26871680 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

