Optogenetic photochemical control of designer K+ channels in mammalian neurons
- PMID: 21525363
- PMCID: PMC3129715
- DOI: 10.1152/jn.00251.2011
Optogenetic photochemical control of designer K+ channels in mammalian neurons
Abstract
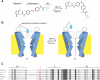
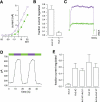
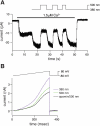
Currently available optogenetic tools, including microbial light-activated ion channels and transporters, are transforming systems neuroscience by enabling precise remote control of neuronal firing, but they tell us little about the role of indigenous ion channels in controlling neuronal function. Here, we employ a chemical-genetic strategy to engineer light sensitivity into several mammalian K(+) channels that have different gating and modulation properties. These channels provide the means for photoregulating diverse electrophysiological functions. Photosensitivity is conferred on a channel by a tethered ligand photoswitch that contains a cysteine-reactive maleimide (M), a photoisomerizable azobenzene (A), and a quaternary ammonium (Q), a K(+) channel pore blocker. Using mutagenesis, we identify the optimal extracellular cysteine attachment site where MAQ conjugation results in pore blockade when the azobenzene moiety is in the trans but not cis configuration. With this strategy, we have conferred photosensitivity on channels containing Kv1.3 subunits (which control axonal action potential repolarization), Kv3.1 subunits (which contribute to rapid-firing properties of brain neurons), Kv7.2 subunits (which underlie "M-current"), and SK2 subunits (which are Ca(2+)-activated K(+) channels that contribute to synaptic responses). These light-regulated channels may be overexpressed in genetically targeted neurons or substituted for native channels with gene knockin technology to enable precise optopharmacological manipulation of channel function.
Figures
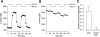


References
-
- Abbott GW. Molecular mechanisms of cardiac voltage-gated potassium channelopathies. Curr Pharm Des 12: 3631–3644, 2006 - PubMed
-
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci 6: 258–266, 2003 - PubMed
-
- Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ, Andrews B S, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA 103: 17414–17419, 2006 - PMC - PubMed
-
- Blaustein RO, Cole PA, Williams C, Miller C. Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat Struct Biol 7: 309–311, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

