IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo
- PMID: 21525380
- PMCID: PMC3098943
- DOI: 10.4049/jimmunol.1100061
IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo
Abstract
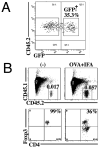
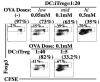
Stimulation of naive mouse CD4(+)Foxp3(-) T cells in the presence of TGF-β results in the induction of Foxp3 expression and T suppressor function. However, Foxp3 expression in these induced regulatory T cells (iTreg) is unstable, raising the possibility that iTreg would not be useful for treatment of autoimmune diseases. To analyze the factors that control the stability of Foxp3 expression in iTreg, we generated OVA-specific iTreg from OT-II Foxp3-GFP knockin mice. Following transfer to normal C57BL/6 mice, OT-II GFP(+) cells maintained high levels of Foxp3 expression for 8 d. However, they rapidly lost Foxp3 expression upon stimulation with OVA in IFA in vivo. This unstable phenotype was associated with a strong methylation of the Treg-specific demethylated region within the Foxp3 locus. Administration of IL-2/anti-IL-2 complexes expanded the numbers of transferred Foxp3(+) iTreg in the absence of Ag challenge. Notably, when the iTreg were stimulated with Ag, treatment with IL-2/anti-IL-2 complexes stabilized Foxp3 expression and resulted in enhanced demethylation of the Treg-specific demethylated region. Conversely, neutralization of IL-2 or disruption of its signaling by deletion of Stat5 diminished the level of Foxp3 expression resulting in decreased suppressor function of the iTreg in vivo. Our data suggest that stimulation with TGF-β in vitro is not sufficient for imprinting T cells with stable expression of Foxp3. Administration of IL-2 in vivo results in stabilization of Foxp3 expression and may prove to be a valuable adjunct for the use of iTreg for the treatment of autoimmune diseases.
Figures





References
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. - PubMed
-
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. - PubMed
-
- Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

