Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo
- PMID: 21525381
- PMCID: PMC3110737
- DOI: 10.4049/jimmunol.1002794
Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo
Abstract
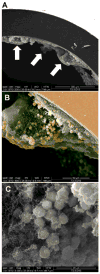
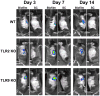
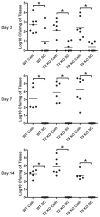
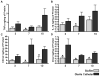
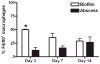
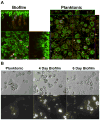
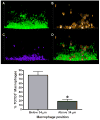
Biofilms are complex communities of bacteria encased in a matrix composed primarily of polysaccharides, extracellular DNA, and protein. Staphylococcus aureus can form biofilm infections, which are often debilitating due to their chronicity and recalcitrance to antibiotic therapy. Currently, the immune mechanisms elicited during biofilm growth and their impact on bacterial clearance remain to be defined. We used a mouse model of catheter-associated biofilm infection to assess the functional importance of TLR2 and TLR9 in the host immune response during biofilm formation, because ligands for both receptors are present within the biofilm. Interestingly, neither TLR2 nor TLR9 impacted bacterial density or inflammatory mediator secretion during biofilm growth in vivo, suggesting that S. aureus biofilms circumvent these traditional bacterial recognition pathways. Several potential mechanisms were identified to account for biofilm evasion of innate immunity, including significant reductions in IL-1β, TNF-α, CXCL2, and CCL2 expression during biofilm infection compared with the wound healing response elicited by sterile catheters, limited macrophage invasion into biofilms in vivo, and a skewing of the immune response away from a microbicidal phenotype as evidenced by decreases in inducible NO synthase expression concomitant with robust arginase-1 induction. Coculture studies of macrophages with S. aureus biofilms in vitro revealed that macrophages successful at biofilm invasion displayed limited phagocytosis and gene expression patterns reminiscent of alternatively activated M2 macrophages. Collectively, these findings demonstrate that S. aureus biofilms are capable of attenuating traditional host proinflammatory responses, which may explain why biofilm infections persist in an immunocompetent host.
Figures








References
-
- Fitzpatrick F, Humphreys H, O’Gara JP. The genetics of staphylococcal biofilm formation--will a greater understanding of pathogenesis lead to better management of device-related infection? Clin Microbiol Infect. 2005;11:967–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

