The pleckstrin homology domain in the SKAP55 adapter protein defines the ability of the adapter protein ADAP to regulate integrin function and NF-kappaB activation
- PMID: 21525391
- PMCID: PMC3108501
- DOI: 10.4049/jimmunol.1002950
The pleckstrin homology domain in the SKAP55 adapter protein defines the ability of the adapter protein ADAP to regulate integrin function and NF-kappaB activation
Abstract
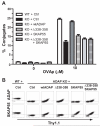
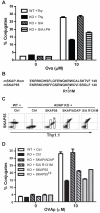
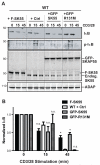
Adhesion and degranulation promoting adapter protein (ADAP) is a multifunctional hematopoietic adapter protein that regulates TCR-dependent increases in both integrin function and activation of the NF-κB transcription factor. Activation of integrin function requires both ADAP and the ADAP-associated adapter Src kinase-associated phosphoprotein of 55 kDa (SKAP55). In contrast, ADAP-mediated regulation of NF-κB involves distinct binding sites in ADAP that promote the inducible association of ADAP, but not SKAP55, with the CARMA1 adapter and the TAK1 kinase. This suggests that the presence or absence of associated SKAP55 defines functionally distinct pools of ADAP. To test this hypothesis, we developed a novel SKAP-ADAP chimeric fusion protein and demonstrated that physical association of ADAP with SKAP55 is both sufficient and necessary for the rescue of integrin function in ADAP-deficient T cells. Similar to wild-type ADAP, the SKAP-ADAP chimera associated with the LFA-1 integrin after TCR stimulation. Although the SKAP-ADAP chimera contains the CARMA1 and TAK1 binding sequences from ADAP, expression of the chimera does not restore NF-κB signaling in ADAP(-/-) T cells. A single point mutation in the pleckstrin homology domain of SKAP55 (R131M) blocks the ability of the SKAP-ADAP chimera to restore integrin function and to associate with LFA-1. However, the R131M mutant was now able to restore NF-κB signaling in ADAP-deficient T cells. We conclude that integrin regulation by ADAP involves the recruitment of ADAP to LFA-1 integrin complexes by the pleckstrin homology domain of SKAP55, and this recruitment restricts the ability of ADAP to interact with the NF-κB signalosome and regulate NF-κB activation.
Figures




References
-
- Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T cell receptor signaling to integrins. Immunol. Rev. 2007;218:65–81. - PubMed
-
- Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Curr. Opin. Immunol. 2006;18:512–516. - PubMed
-
- Kane LP, Lin J, Weiss A. It’s all Rel-ative: NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 2002;23:413–420. - PubMed
-
- Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. - PubMed
-
- Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat. Rev. Immunol. 2006;6:67–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

