Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells
- PMID: 21525398
- PMCID: PMC3861895
- DOI: 10.1126/scitranslmed.3002207
Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells
Abstract
Although advanced-stage melanoma patients have a median survival of less than a year, adoptive T cell therapy can induce durable clinical responses in some patients. Successful adoptive T cell therapy to treat cancer requires engraftment of antitumor T lymphocytes that not only retain specificity and function in vivo but also display an intrinsic capacity to survive. To date, adoptively transferred antitumor CD8(+) T lymphocytes (CTLs) have had limited life spans unless the host has been manipulated. To generate CTLs that have an intrinsic capacity to persist in vivo, we developed a human artificial antigen-presenting cell system that can educate antitumor CTLs to acquire both a central memory and an effector memory phenotype as well as the capacity to survive in culture for prolonged periods of time. We examined whether antitumor CTLs generated using this system could function and persist in patients. We showed that MART1-specific CTLs, educated and expanded using our artificial antigen-presenting cell system, could survive for prolonged periods in advanced-stage melanoma patients without previous conditioning or cytokine treatment. Moreover, these CTLs trafficked to the tumor, mediated biological and clinical responses, and established antitumor immunologic memory. Therefore, this approach may broaden the availability of adoptive cell therapy to patients both alone and in combination with other therapeutic modalities.
Figures
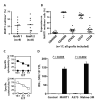
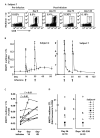

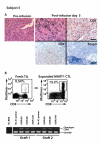
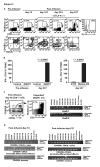
References
-
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 - PMC - PubMed
-
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

