Identification of cytosolic phosphodiesterases in the erythrocyte: a possible role for PDE5
- PMID: 21525805
- PMCID: PMC3366467
- DOI: 10.12659/msm.881763
Identification of cytosolic phosphodiesterases in the erythrocyte: a possible role for PDE5
Abstract
Background: Within erythrocytes (RBCs), cAMP levels are regulated by phosphodiesterases (PDEs). Increases in cAMP and ATP release associated with activation of β-adrenergic receptors (βARs) and prostacyclin receptors (IPRs) are regulated by PDEs 2, 4 and PDE 3, respectively. Here we establish the presence of cytosolic PDEs in RBCs and determine a role for PDE5 in regulating levels of cGMP.
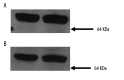
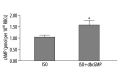
Material/methods: Purified cytosolic proteins were obtained from isolated human RBCs and western analysis was performed using antibodies against PDEs 3A, 4 and 5. Rabbit RBCs were incubated with dbcGMP, a cGMP analog, to determine the effect of cGMP on cAMP levels. To determine if cGMP affects receptor-mediated increases in cAMP, rabbit RBCs were incubated with dbcGMP prior to addition of isoproterenol (ISO), a βAR receptor agonist. To demonstrate that endogenous cGMP produces the same effect, rabbit and human RBCs were incubated with SpNONOate (SpNO), a nitric oxide donor, and YC1, a direct activator of soluble guanylyl cyclase (sGC), in the absence and presence of a selective PDE5 inhibitor, zaprinast (ZAP).
Results: Western analysis identified PDEs 3A, 4D and 5A. dbcGMP produced a concentration dependent increase in cAMP and ISO-induced increases in cAMP were potentiated by dbcGMP. In addition, incubation with YC1 and SpNO in the presence of ZAP potentiated βAR-induced increases in cAMP.
Conclusions: PDEs 2, 3A and 5 are present in the cytosol of human RBCs. PDE5 activity in RBCs regulates cGMP levels. Increases in intracellular cGMP augment cAMP levels. These studies suggest a novel role for PDE5 in erythrocytes.
Figures





References
-
- Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit. 2001;7:669–74. - PubMed
-
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. - PubMed
-
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

