Efficacy of interferon alpha-2b with or without ribavirin in thalassemia major patients with chronic hepatitis C virus infection: A randomized, double blind, controlled, parallel group trial
- PMID: 21526103
- PMCID: PMC3082835
Efficacy of interferon alpha-2b with or without ribavirin in thalassemia major patients with chronic hepatitis C virus infection: A randomized, double blind, controlled, parallel group trial
Abstract
Background: The aim of this study was to evaluate the effectiveness of monotherapy with interferon alpha-2b and combination therapy with interferon alpha-2b plus ribavirin on chronic hepatitis C infection in thalassaemic patients.
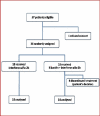
Methods: In parallel group randomized, double blind, controlled trial, 32 thalassaemic patients with chronic hepatitis C infection completed the study. In a random fashion, one group was treated with three million units of interferon alpha-2b three times a week plus ribavirin (800-1200 mg daily). The second group received interferon alpha-2b alone. Treatment duration was 24-48 weeks. Primary efficacy variables were HCV RNA after treatment and sustained viral response (SVR) six months after treatment.
Results: The mean age of patients was 22 ± 7.4 years; 19 (59.4%) were male and 13 (40.6) were female. At the end of treatment, no statistically significant differences were found between the groups in HCV RNA and AST. The proportion of patients with SVR six months after treatment was significantly greater in the monotherapy group (90.9%) than in the combination therapy group (44.4%; p = 0.049). A significant difference in mean of ALT was also obtained at the end of treatment between monotherapy and combination therapy groups (30.4 ± 19.2 and 60.1 ± 48.9, respectively; p = 0.02). Response rates were not associated with genotype and severity of hepatitis C infection in both groups.
Conclusions: These results suggest that monotherapy may be considered as the first-line therapy in patients with thalassemia.
Keywords: Hepacivirus; Interferons; Ribavirin; Thalassemia.
Conflict of interest statement
Figures

Similar articles
-
Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial.Lancet. 2014 Aug 2;384(9941):414-26. doi: 10.1016/S0140-6736(14)60538-9. Epub 2014 Jun 4. Lancet. 2014. PMID: 24907224 Clinical Trial.
-
Interferon-alpha-2b plus ribavirin: a review of its use in the management of chronic hepatitis C.Drugs. 2002;62(3):507-56. doi: 10.2165/00003495-200262030-00009. Drugs. 2002. PMID: 11827565 Review.
-
Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group.Lancet. 1998 Jan 10;351(9096):83-7. doi: 10.1016/s0140-6736(97)06088-1. Lancet. 1998. PMID: 9439491 Clinical Trial.
-
Triple combination of interferon alpha-2b, ribavirin, and amantadine for treatment of chronic hepatitis C.J Clin Gastroenterol. 2003 May-Jun;36(5):427-30. doi: 10.1097/00004836-200305000-00014. J Clin Gastroenterol. 2003. PMID: 12702987
-
Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b.Drugs. 2010;70(2):147-65. doi: 10.2165/11531990-000000000-00000. Drugs. 2010. PMID: 20108989 Review.
Cited by
-
Safety and Efficacy of Adding Ribavirin to Interferon or Peginterferon in Treatment of Hepatitis C Infection in Patients With Thalassemia: A Systematic Review on Randomized Controlled Trials.Hepat Mon. 2016 Mar 6;16(3):e28537. doi: 10.5812/hepatmon.28537. eCollection 2016 Mar. Hepat Mon. 2016. PMID: 27226796 Free PMC article. Review.
-
Effects of silybum marianum on patients with chronic hepatitis C.J Res Med Sci. 2011 Mar;16(3):287-90. J Res Med Sci. 2011. PMID: 22091246 Free PMC article.
References
-
- Viral hepatitis. Centers for Disease Control and Prevention. Available at: www.cdc.gov/ncidod/diseases/hepatitis/slideset/index.htm. Accessed November 18, 2009.
-
- Kalantari H, Kazemi F, Minakari M. Efficacy of triple therapy with interferon alpha-2b, ribavirin and amantadine in the treatment of naïve patients with chronic hepatitis C. J Res Med Sci. 2007;12(4):178–85.
-
- Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Thalassemia Clinical Research Network. Complications of beta-thalassemia major in North America. Blood. 2004;104(1):34–9. - PubMed
-
- Bourliere M, Halfon P, Portal I. Treatment of chronic hepatitis C in special groups. Gastroenterol Clin Biol. 2002;26 Spec No 2:B238–47. - PubMed
LinkOut - more resources
Full Text Sources
