Mature peripheral RPE cells have an intrinsic capacity to proliferate; a potential regulatory mechanism for age-related cell loss
- PMID: 21526120
- PMCID: PMC3081302
- DOI: 10.1371/journal.pone.0018921
Mature peripheral RPE cells have an intrinsic capacity to proliferate; a potential regulatory mechanism for age-related cell loss
Abstract
Background: Mammalian peripheral retinal pigmented epithelium (RPE) cells proliferate throughout life, while central cells are senescent. It is thought that some peripheral cells migrate centrally to correct age-related central RPE loss.
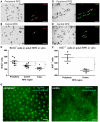
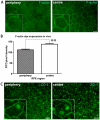
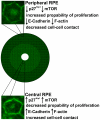
Methodology/principal findings: We ask whether this proliferative capacity is intrinsic to such cells and whether cells located centrally produce diffusible signals imposing senescence upon the former once migrated. We also ask whether there are regional differences in expression patterns of key genes involved in these features between the centre and the periphery in vivo and in vitro. Low density RPE cultures obtained from adult mice revealed significantly greater levels of proliferation when derived from peripheral compared to central tissue, but this significance declined with increasing culture density. Further, exposure to centrally conditioned media had no influence on proliferation in peripheral RPE cell cultures at the concentrations examined. Central cells expressed significantly higher levels of E-Cadherin revealing a tighter cell adhesion than in the peripheral regions. Fluorescence-labelled staining for E-Cadherin, F-actin and ZO-1 in vivo revealed different patterns with significantly increased expression on central RPE cells than those in the periphery or differences in junctional morphology. A range of other genes were investigated both in vivo and in vitro associated with RPE proliferation in order to identify gene expression differences between the centre and the periphery. Specifically, the cell cycle inhibitor p27(Kip1) was significantly elevated in central senescent regions in vivo and mTOR, associated with RPE cell senescence, was significantly elevated in the centre in comparison to the periphery.
Conclusions: These data show that the proliferative capacity of peripheral RPE cells is intrinsic and cell-autonomous in adult mice. These differences between centre and periphery are reflected in distinct patterns in junctional markers. The regional proliferation differences may be inversely dependent to cell-cell contact.
Conflict of interest statement
Figures




References
-
- Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286–1295. - PubMed
-
- Elman MJ, Fine SL, Murphy RP, Patz A, Auer C. The natural history of serous retinal pigment epithelium detachment in patients with age-related macular degeneration. Ophthalmology. 1986;93:224–230. - PubMed
-
- Lai YL, Rana MW. A study of photoreceptor-retinal pigment epithelium complex: age-related changes in monkeys. Proc Soc Exp Biol Med. 1986;181:371–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

