Unrepaired clustered DNA lesions induce chromosome breakage in human cells
- PMID: 21527720
- PMCID: PMC3100920
- DOI: 10.1073/pnas.1016045108
Unrepaired clustered DNA lesions induce chromosome breakage in human cells
Abstract
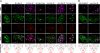
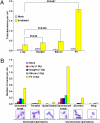
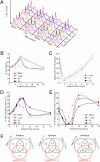
Clustered DNA damage induced by ionizing radiation is refractory to repair and may trigger carcinogenic events for reasons that are not well understood. Here, we used an in situ method to directly monitor induction and repair of clustered DNA lesions in individual cells. We showed, consistent with biophysical modeling, that the kinetics of loss of clustered DNA lesions was substantially compromised in human fibroblasts. The unique spatial distribution of different types of DNA lesions within the clustered damages, but not the physical location of these damages within the subnuclear domains, determined the cellular ability to repair the damage. We then examined checkpoint arrest mechanisms and yield of gross chromosomal aberrations. Induction of nonrepairable clustered damage affected only G2 accumulation but not the early G2/M checkpoint. Further, cells that were released from the G2/M checkpoint with unrepaired clustered damage manifested a spectrum of chromosome aberrations in mitosis. Difficulties associated with clustered DNA damage repair and checkpoint release before the completion of clustered DNA damage repair appear to promote genome instability that may lead to carcinogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nikjoo H, O'Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156:577–583. - PubMed
-
- Goodhead DT. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. - PubMed
-
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. - PubMed
-
- Brenner DJ, Ward JF. Constraints on energy deposition and target size of multiply damaged sites associated with DNA double-strand breaks. Int J Radiat Biol. 1992;61:737–748. - PubMed
-
- Blaisdell JO, Harrison L, Wallace SS. Base excision repair processing of radiation-induced clustered DNA lesions. Radiat Prot Dosimetry. 2001;97:25–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

