Hepatic stellate cells require a stiff environment for myofibroblastic differentiation
- PMID: 21527725
- PMCID: PMC3129929
- DOI: 10.1152/ajpgi.00412.2010
Hepatic stellate cells require a stiff environment for myofibroblastic differentiation
Abstract
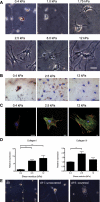
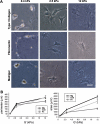
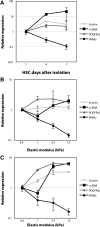
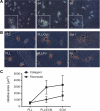
The myofibroblastic differentiation of hepatic stellate cells (HSC) is a critical event in liver fibrosis and is part of the final common pathway to cirrhosis in chronic liver disease from all causes. The molecular mechanisms driving HSC differentiation are not fully understood. Because macroscopic tissue stiffening is a feature of fibrotic disease, we hypothesized that mechanical properties of the underlying matrix are a principal determinant of HSC activation. Primary rat HSC were cultured on inert polyacrylamide supports of variable but precisely defined shear modulus (stiffness) coated with different extracellular matrix proteins or poly-L-lysine. HSC differentiation was determined by cell morphology, immunofluorescence staining, and gene expression. HSC became progressively myofibroblastic as substrate stiffness increased on all coating matrices, including Matrigel. The degree rather than speed of HSC activation correlated with substrate stiffness, with cells cultured on supports of intermediate stiffness adopting stable intermediate phenotypes. Quiescent cells on soft supports were able to undergo myofibroblastic differentiation with exposure to stiff supports. Stiffness-dependent differentiation required adhesion to matrix proteins and the generation of mechanical tension. Transforming growth factor-β treatment enhanced differentiation on stiff supports, but was not required. HSC differentiate to myofibroblasts in vitro primarily as a function of the physical rather than the chemical properties of the substrate. HSC require a mechanically stiff substrate, with adhesion to matrix proteins and the generation of mechanical tension, to differentiate. These findings suggest that alterations in liver stiffness are a key factor driving the progression of fibrosis.
Figures


References
-
- Basu A, Wen Q, Lubensky T, Janmey P, Yodh A. Non-affine displacements in flexible polymer gels. Macromol. 44: 1671–1679, 2011
-
- Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol 18: 472–481, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

