Microbial Pathogens in the Fungal Kingdom
- PMID: 21528015
- PMCID: PMC3081590
- DOI: 10.1016/j.fbr.2011.01.003
Microbial Pathogens in the Fungal Kingdom
Abstract
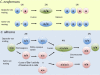
The fungal kingdom is vast, spanning ~1.5 to as many as 5 million species diverse as unicellular yeasts, filamentous fungi, mushrooms, lichens, and both plant and animal pathogens. The fungi are closely aligned with animals in one of the six to eight supergroups of eukaryotes, the opisthokonts. The animal and fungal kingdoms last shared a common ancestor ~1 billion years ago, more recently than other groups of eukaryotes. As a consequence of their close evolutionary history and shared cellular machinery with metazoans, fungi are exceptional models for mammalian biology, but prove more difficult to treat in infected animals. The last common ancestor to the fungal/metazoan lineages is thought to have been unicellular, aquatic, and motile with a posterior flagellum, and certain extant species closely resemble this hypothesized ancestor. Species within the fungal kingdom were traditionally assigned to four phyla, including the basal fungi (Chytridiomycota, Zygomycota) and the more recently derived monophyletic lineage, the dikarya (Ascomycota, Basidiomycota). The fungal tree of life project has revealed that the basal lineages are polyphyletic, and thus there are as many as eight to ten fungal phyla. Fungi that infect vertebrates are found in all of the major lineages, and virulence arose multiple times independently. A sobering recent development involves the species Batrachochytrium dendrobatidis from the basal fungal phylum, the Chytridiomycota, which has emerged to cause global amphibian declines and extinctions. Genomics is revolutionizing our view of the fungal kingdom, and genome sequences for zygomycete pathogens (Rhizopus, Mucor), skin-associated fungi (dermatophytes, Malassezia), and the Candida pathogenic species clade promise to provide insights into the origins of virulence. Here we survey the diversity of fungal pathogens and illustrate key principles revealed by genomics involving sexual reproduction and sex determination, loss of conserved pathways in derived fungal lineages that are retained in basal fungi, and shared and divergent virulence strategies of successful human pathogens, including dimorphic and trimorphic transitions in form. The overarching conclusion is that fungal pathogens of animals have arisen repeatedly and independently throughout the fungal tree of life, and while they share general properties, there are also unique features to the virulence strategies of each successful microbial pathogen.
Figures


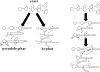
References
-
- Anzawa K, Kawasaki M, Mochizuki T, Ishizaki H. Successful mating of Trichophyton rubrum with Arthroderma simii. Med Mycol. 2010;48:629–634. - PubMed
-
- Bakkeren G, Kamper J, Schirawski J. Sex in smut fungi: Structure, function and evolution of mating-type complexes. Fungal Genet Biol. 2008;45(Suppl 1):S15–21. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
