Surface engineering of iron oxide nanoparticles for targeted cancer therapy
- PMID: 21528865
- PMCID: PMC3192288
- DOI: 10.1021/ar2000277
Surface engineering of iron oxide nanoparticles for targeted cancer therapy
Abstract
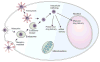
Nanotechnology provides a flexible platform for the development of effective therapeutic nanomaterials that can interact specifically with a target in a biological system and provoke a desired response. Of the nanomaterials studied, iron oxide nanoparticles have emerged as one of top candidates for cancer therapy. Their intrinsic superparamagnetism enables noninvasive magnetic resonance imaging (MRI), and their biodegradability is advantageous for in vivo applications. A therapeutic superparamagnetic iron oxide nanoparticle (SPION) typically consists of three primary components: an iron oxide nanoparticle core that serves as both a carrier for therapeutics and contrast agent for MRI, a coating on the iron oxide nanoparticle that promotes favorable interactions between the SPION and the biological system, and a therapeutic payload that performs the designated function in vivo. Often, the design may include a targeting ligand that recognizes the receptors over-expressed on the exterior surface of cancer cells. The body is a highly complex system that imposes multiple physiological and cellular barriers to foreign objects. Thus, the success of a therapeutic SPION largely relies on the design of the iron oxide core to ensure its detection in MRI and the coatings that allow the nanoparticles to bypass these barriers. Strategies to bypass the physiological barriers, such as liver, kidneys, and spleen, involve tuning the overall size and surface chemistry of the SPION to maximize blood half-life and facilitate the navigation in the body. Strategies to bypass cellular barriers include the use of targeting agents to maximize uptake of the SPION by cancer cells and the employment of materials that promote desired intracellular trafficking and enable controlled drug release. The payload can be genes, proteins, chemotherapy drugs, or a combination of these molecules. Each type of therapeutic molecule requires a specific coating design to maximize the loading and to achieve effective delivery and release. In this Account, we discuss the primary design parameters in developing therapeutic SPIONs with a focus on surface coating design to overcome the barriers imposed by the body's defense system. We provide examples of how these design parameters have been implemented to produce SPIONs for specific therapeutic applications. Although there are still challenges to be addressed, SPIONs show great promise in the successful diagnosis and treatment of the most devastating cancers. Once the critical design parameters have been optimized, these nanoparticles, combined with imaging modalities, can serve as truly multifunctional theranostic agents that not only perform a therapeutic function but also provide instant clinical feedback, allowing the physician to adjust the treatment plan.
Figures





References
-
- Jordan A, Scholz R, Maier-Hauff K, van Landeghem FKH, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neuro-Oncol. 2006;78:7–14. - PubMed
-
- Day ES, Morton JG, West JL. Nanoparticles for thermal cancer therapy. J Biomech Eng. 2009;131:074001. - PubMed
-
- Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13:95–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

