Comparison of human optimized bacterial luciferase, firefly luciferase, and green fluorescent protein for continuous imaging of cell culture and animal models
- PMID: 21529093
- PMCID: PMC3094131
- DOI: 10.1117/1.3564910
Comparison of human optimized bacterial luciferase, firefly luciferase, and green fluorescent protein for continuous imaging of cell culture and animal models
Abstract
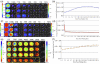
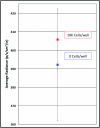
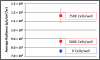
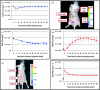
Bioluminescent and fluorescent reporter systems have enabled the rapid and continued growth of the optical imaging field over the last two decades. Of particular interest has been noninvasive signal detection from mammalian tissues under both cell culture and whole animal settings. Here we report on the advantages and limitations of imaging using a recently introduced bacterial luciferase (lux) reporter system engineered for increased bioluminescent expression in the mammalian cellular environment. Comparison with the bioluminescent firefly luciferase (Luc) system and green fluorescent protein system under cell culture conditions demonstrated a reduced average radiance, but maintained a more constant level of bioluminescent output without the need for substrate addition or exogenous excitation to elicit the production of signal. Comparison with the Luc system following subcutaneous and intraperitoneal injection into nude mice hosts demonstrated the ability to obtain similar detection patterns with in vitro experiments at cell population sizes above 2.5 × 10(4) cells but at the cost of increasing overall image integration time.
Figures


References
-
- Choy G., O’Connor S., Diehn F., Costouros N., Alexander H., Choyke P., and Libutti S., “Comparison of noninvasive fluorescent and bioluminescent small animal optical imaging,” BioTechniques 35(5), 1022–1031 (2003). - PubMed
-
- Ando Y., Niwa K., Yamada N., Enomoto T., Irie T., Kubota H., Ohmiya Y., and Akiyama H., “Firefly bioluminescence quantum yield and colour change by pH-sensitive green emission,” Nature Photon. 2(1), 44–47 (2007).10.1038/nphoton.2007.251 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

