Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study
- PMID: 21529365
- PMCID: PMC3108934
- DOI: 10.1186/1475-2875-10-107
Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study
Abstract
Background: Presumptive treatment of all febrile patients with anti-malarials leads to massive over-treatment. The aim was to assess the effect of implementing malaria rapid diagnostic tests (mRDTs) on prescription of anti-malarials in urban Tanzania.
Methods: The design was a prospective collection of routine statistics from ledger books and cross-sectional surveys before and after intervention in randomly selected health facilities (HF) in Dar es Salaam, Tanzania. The participants were all clinicians and their patients in the above health facilities. The intervention consisted of training and introduction of mRDTs in all three hospitals and in six HF. Three HF without mRDTs were selected as matched controls. The use of routine mRDT and treatment upon result was advised for all patients complaining of fever, including children under five years of age. The main outcome measures were: (1) anti-malarial consumption recorded from routine statistics in ledger books of all HF before and after intervention; (2) anti-malarial prescription recorded during observed consultations in cross-sectional surveys conducted in all HF before and 18 months after mRDT implementation.
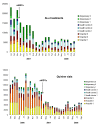
Results: Based on routine statistics, the amount of artemether-lumefantrine blisters used post-intervention was reduced by 68% (95%CI 57-80) in intervention and 32% (9-54) in control HF. For quinine vials, the reduction was 63% (54-72) in intervention and an increase of 2.49 times (1.62-3.35) in control HF. Before-and-after cross-sectional surveys showed a similar decrease from 75% to 20% in the proportion of patients receiving anti-malarial treatment (Risk ratio 0.23, 95%CI 0.20-0.26). The cluster randomized analysis showed a considerable difference of anti-malarial prescription between intervention HF (22%) and control HF (60%) (Risk ratio 0.30, 95%CI 0.14-0.70). Adherence to test result was excellent since only 7% of negative patients received an anti-malarial. However, antibiotic prescription increased from 49% before to 72% after intervention (Risk ratio 1.47, 95%CI 1.37-1.59).
Conclusions: Programmatic implementation of mRDTs in a moderately endemic area reduced drastically over-treatment with anti-malarials. Properly trained clinicians with adequate support complied with the recommendation of not treating patients with negative results. Implementation of mRDT should be integrated hand-in-hand with training on the management of other causes of fever to prevent irrational use of antibiotics.
Figures




References
-
- World Health Organization. Guidelines for the Treatment of Malaria. Geneva, Switzerland. 2006.
-
- D'Acremont V, Lengeler C, Genton B. Massive over-diagnosis of malaria in Sub-Saharan Africa: time to review blanket treatment of underfives. Abstract book of the 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Philadelphia, USA. 2007. pp. 4–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

