ClpX(P) generates mechanical force to unfold and translocate its protein substrates
- PMID: 21529717
- PMCID: PMC3686100
- DOI: 10.1016/j.cell.2011.04.010
ClpX(P) generates mechanical force to unfold and translocate its protein substrates
Abstract
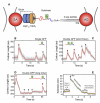
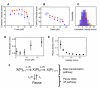
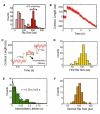
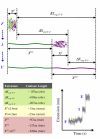
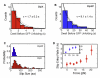
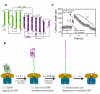
AAA(+) unfoldases denature and translocate polypeptides into associated peptidases. We report direct observations of mechanical, force-induced protein unfolding by the ClpX unfoldase from E. coli, alone, and in complex with the ClpP peptidase. ClpX hydrolyzes ATP to generate mechanical force and translocate polypeptides through its central pore. Threading is interrupted by pauses that are found to be off the main translocation pathway. ClpX's translocation velocity is force dependent, reaching a maximum of 80 aa/s near-zero force and vanishing at around 20 pN. ClpX takes 1, 2, or 3 nm steps, suggesting a fundamental step-size of 1 nm and a certain degree of intersubunit coordination. When ClpX encounters a folded protein, it either overcomes this mechanical barrier or slips on the polypeptide before making another unfolding attempt. Binding of ClpP decreases the slip probability and enhances the unfolding efficiency of ClpX. Under the action of ClpXP, GFP unravels cooperatively via a transient intermediate.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Protease power strokes force proteins to unfold.Cell. 2011 Apr 29;145(3):339-40. doi: 10.1016/j.cell.2011.04.007. Cell. 2011. PMID: 21529709 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

