Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein
- PMID: 21529875
- PMCID: PMC3107944
- DOI: 10.1016/j.virol.2011.04.002
Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein
Abstract
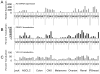
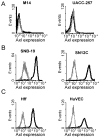
In a bioinformatics-based screen for cellular genes that enhance Zaire ebolavirus (ZEBOV) transduction, AXL mRNA expression strongly correlated with ZEBOV infection. A series of cell lines and primary cells were identified that require Axl for optimal ZEBOV entry. Using one of these cell lines, we identified ZEBOV entry events that are Axl-dependent. Interactions between ZEBOV-GP and the Axl ectodomain were not detected in immunoprecipitations and reduction of surface-expressed Axl by RNAi did not alter ZEBOV-GP binding, providing evidence that Axl does not serve as a receptor for the virus. However, RNAi knock down of Axl reduced ZEBOV pseudovirion internalization and α-Axl antisera inhibited pseudovirion fusion with cellular membranes. Consistent with the importance of Axl for ZEBOV transduction, Axl transiently co-localized on the surface of cells with ZEBOV virus particles and was internalized during virion transduction. In total, these findings indicate that endosomal uptake of filoviruses is facilitated by Axl.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179–192. - PubMed
-
- Baribaud F, Doms RW, Pohlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin Ther Targets. 2002;6:423–431. - PubMed
-
- Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, Bartram CR, Janssen JW. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14:2619–2631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

