A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: a Gynecologic Oncology Group Study
- PMID: 21529904
- PMCID: PMC3110746
- DOI: 10.1016/j.ygyno.2011.03.013
A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: a Gynecologic Oncology Group Study
Abstract
Objective: To compare the recurrence-free interval (RFI) and safety profile in patients with completely resected high-risk early-stage ovarian cancer treated with intravenous (IV) carboplatin and paclitaxel with or without maintenance low-dose paclitaxel for 24 weeks.
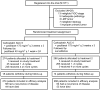
Methods: Eligibility was limited to patients with stage IA/B (grade 3 or clear cell), all IC or II epithelial ovarian cancer. All patients were to receive carboplatin AUC 6 and paclitaxel 175 mg/m² q3 weeks × 3 courses with random assignment to either observation or maintenance paclitaxel 40 mg/m²/week × 24 weeks. Recurrence required clinical or radiological evidence of new tumor.
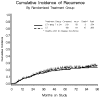
Results: There were 571 patients enrolled onto this study, of whom 29 were deemed ineligible due to inappropriate stage or pathology, leaving 542 patients. At least 3 cycles of treatment were administered to 524/542 (97%) of patients, and among those assigned to maintenance paclitaxel, 80% completed the regimen. The incidence of grade 2 or worse peripheral neuropathy (15.5% vs. 6%), infection/fever (19.9% vs. 8.7%), and dermatologic events (70.8% vs. 52.1%) was higher on the maintenance regimen (p<0.001). The cumulative probability of recurring within 5 years for the maintenance paclitaxel regimen is 20% vs. 23% for surveillance (hazard ratio 0.807; 95% CI: 0.565-1.15). The probability of surviving 5 years was 85.4% and 86.2%, respectively.
Conclusion: Maintenance paclitaxel at 40 mg/m²/week × 24 weeks added to standard dose AUC6 and paclitaxel 175 mg/m² × 3 doses provides no significant increase in RFI.
Trial registration: ClinicalTrials.gov NCT00003644.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors wish to report that there are no conflicts of interest with the exception of Dr. Helen Michael who reports that, although she is not aware of any financial relationships involving this study, she does hold stocks and/or bonds in the following companies: Johnson & Johnson, Amgen, Teva Pharmaceutical Industries ADR, Medtronic, Roche Holding ADR, Eli Lilly & Co, Pfizer, Abbott Laboratories, Becton Dickinson. Dr. Michael also reports that she does not select or manage her investments but rather an asset management company does that for her.
Figures
Comment in
-
Early stage ovarian cancers: angiogenesis inhibition and clear cell cancers.Gynecol Oncol. 2011 Nov;123(2):426; author reply 427. doi: 10.1016/j.ygyno.2011.07.036. Epub 2011 Aug 16. Gynecol Oncol. 2011. PMID: 21846574 No abstract available.
References
-
- Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. N Engl J Med. 1990;322:1021–27. - PubMed
-
- Young RC, Brady MF, Nieberg RM, et al. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:4350–55. - PubMed
-
- Bolis G, Colombo N, Pecorelli S, et al. Adjuvant treatment for early epithelial ovarian cancer results of two randomized clinical trials comparing cisplatin to no further treatment of chronic phosphate (32P). GICOG: Gruppo Interregionale Collaborativo in Ginecologia Oncologica. Ann Oncol. 1995;6:887–93. - PubMed
-
- Vergote IB, Vergote-De Vos LN, Abeler VM, et al. Randomized trial comparing cisplatin with radioactive phosphorus or whole-abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer. 1992;69:741–49. - PubMed
-
- Trimbos JB, Parmar M, Vergote I, et al. International collaborative ovarian neoplasm trial I and adjuvant chemotherapy in ovarian neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;5:105–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases